Petty RE, Laxer RM, Lindsley CB, et al. Textbook of pediatric rheumatology. 8th ed. Philadelphia: Elsevier, Inc 2020.
Лыскина Г.А. Системные васкулиты. In: Геппе Н.А., Подчерняева Н.С., Лыскина Г.А., eds. Руководство по детской ревматологии. Москва: ГЭОТАР-Медиа 2011:507–99.
Российское общество ангиологов и сосудистых хирургов, Ассоциация сердечно-сосудистых хирургов России, Российское научное общество рентгенэндоваскулярных хирургов и интервенционных радиологов, et al. Национальные рекомендации по ведению пациентов с заболеваниями брахиоцефальных артерий.; Российский согласительный документ. Ангиология и сосудистая хирургия. 2013;19:1–70.
Paediatric Immunology, Hematology and Rheumatology Unit, Hôpital Necker - Enfants Malades, Paris, France, Aeschlimann FA, Division of Paediatrics, Kantonsspital Winterthur, Winterthur, Switzerland, et al. Childhood-onset Takayasu Arteritis. Eur J Rheumatol. 2020;7:58–66. doi: 10.5152/eurjrheum.2019.19195
Баранов А.А., Алексеева Е.И. Детская ревматология. Атлас. 2nd ed. Москва: ПедиатрЪ 2015.
Di Santo M, Stelmaszewski EV, Villa A. Takayasu arteritis in paediatrics. Cardiol Young. 2018;28:354–61. doi: 10.1017/S1047951117001998
Brunner J, Feldman BM, Tyrrell PN, et al. Takayasu arteritis in children and adolescents. Rheumatol Oxf Engl. 2010;49:1806–14. doi: 10.1093/rheumatology/keq167
Aeschlimann FA, Eng SWM, Sheikh S, et al. Childhood Takayasu arteritis: disease course and response to therapy. Arthritis Res Ther. 2017;19:255. doi: 10.1186/s13075-017-1452-4
Pískovský T, Hladík M, Kosňovská L, et al. Takayasu arteritis in a 10-month-old boy. VASA Z Gefasskrankheiten. 2013;42:134–8. doi: 10.1024/0301-1526/a000258
Singh N, Hughes M, Sebire N, et al. Takayasu arteritis in infancy. Rheumatol Oxf Engl. 2013;52:2093–5. doi: 10.1093/rheumatology/ket109
Liu H, Sun L, Upadhyaya RS, et al. Case report: Takayasu arteritis in a 3-month-old Chinese girl. Medicine (Baltimore). 2018;97:e12637. doi: 10.1097/MD.0000000000012637
Higaki R, Miyazaki A, Tajiri Y, et al. Continuous infusion of lipo-prostaglandin E1 for Takayasu’s arteritis with heart failure in an 11-month-old baby: a case report. J Med Case Reports. 2018;12:266. doi: 10.1186/s13256-018-1769-x
Hata A, Noda M, Moriwaki R, et al. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. 1996;54 Suppl:S155-163. doi: 10.1016/s0167-5273(96)02813-6
Баранов А.А., Алексеева Е.И., editors. Ревматические болезни у детей. Москва: ПедиатрЪ 2016.
Лыскина Г.А., Костина Ю.О. Диагностика и лечение неспецифического аортоартериита у детей (обзор). Вестник Смоленской Государственной Медицинской Академии. 2017;16:183–6.
de Graeff N, Groot N, Brogan P, et al. European consensus-based recommendations for the diagnosis and treatment of rare paediatric vasculitides - the SHARE initiative. Rheumatol Oxf Engl. 2019;58:656–71. doi: 10.1093/rheumatology/key322
Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798–806. doi: 10.1136/ard.2009.116657
Seringec Akkececi N, Yildirim Cetin G, Gogebakan H, et al. The C-Reactive Protein/Albumin Ratio and Complete Blood Count Parameters as Indicators of Disease Activity in Patients with Takayasu Arteritis. Med Sci Monit Int Med J Exp Clin Res. 2019;25:1401–9. doi: 10.12659/MSM.912495
Maz M, Chung SA, Abril A, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol Hoboken NJ. 2021;73:1349–65. doi: 10.1002/art.41774
Подзолкова В.А. и др. Артериит Такаясу у детей: особенности клинического течения в дебюте болезни //Доктор. Ру. – 2022. – Т. 21. – №. 3. – С. 28-33.
Eleftheriou D, Varnier G, Dolezalova P, et al. Takayasu arteritis in childhood: retrospective experience from a tertiary referral centre in the United Kingdom. Arthritis Res Ther. 2015;17. doi: 10.1186/s13075-015-0545-1
Klein A, Molad Y. Hematological Manifestations among Patients with Rheumatic Diseases. Acta Haematol. 2021;144:403–12. doi: 10.1159/000511759
Soufla A., Tsiara S. Thrombotic events in inflammatory rheumatological diseases //Rheumatology Quarterly. – 2024.
Leisring J, Brodsky SV, Parikh SV. Clinical Evaluation and Management of Thrombotic Microangiopathy. Arthritis Rheumatol. 2024;76:153–65. doi: 10.1002/art.42681
A resident’s guide to pediatric rheumatology 4 th Revised Edition – 2019. URL: https://renaissance.stonybrookmedicine.edu/sites/default/files/2019_Revised_Residents_Guide__FINAL.pdf.
Fareed J, Iqbal O, Cunanan J, et al. Changing trends in anti-coagulant therapies. Are heparins and oral anti-coagulants challenged? Int Angiol J Int Union Angiol. 2008;27:176–92.
Fareed J, Hoppensteadt DA, Fareed D, et al. Survival of heparins, oral anticoagulants, and aspirin after the year 2010. Semin Thromb Hemost. 2008;34:58–73. doi: 10.1055/s-2008-1066025
Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S-e801S. doi: 10.1378/chest.11-2308
Tarango C, Manco-Johnson MJ. Pediatric Thrombolysis: A Practical Approach. Front Pediatr. 2017;5:260. doi: 10.3389/fped.2017.00260
Wang X, Dang A, Lv N, et al. Inflammation Is Associated With Platelet Coagulation Function Rather Than Enzymatic Coagulation Function in Patients With Takayasu Arteritis. Int Heart J. 2017;58:589–92. doi: 10.1536/ihj.16-533
Тепаев РФ. Синдром диссеминированного внутрисосудистого свертывания у детей. Диагностика и лечение. Педиатрическая Фармакология. 2010;7:27–31.
Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187–93. doi: 10.1136/archdischild-2016-311053
Ameri A, Anderson CM, Smith JH, et al. Thromboembolic Complications in a Pediatric Patient Population: Treatment with Direct Oral Anticoagulants. Monitoring of Treatment Efficiency with D-Dimer Levels and Safety Profile By Thromboelastogram. Blood. 2021;138:4270–4270. doi: 10.1182/blood-2021-146948
Oren H, Cingöz I, Duman M, et al. Disseminated intravascular coagulation in pediatric patients: clinical and laboratory features and prognostic factors influencing the survival. Pediatr Hematol Oncol. 2005;22:679–88. doi: 10.1080/08880010500278749
Sepúlveda M P, Salgado U A, Barriga G J, et al. Usefulness of the thromboelastogram in children: correlation with habitual coagulation tests. Rev Chil Pediatr. 2019;90:617–23. doi: 10.32641/rchped.v90i6.930
Геморрагические и тромботические заболевания и синдромы у детей и подростков: патогенез, клиника, диагностика, терапия и профилактика : монография / Б.И. Кузник, В.Г. Стуров, Н.Ю. Левшин [и др.]. – 2-е изд., перераб. и доп. – Новосибирск : Наука, 2018. – 524 с.
Verma A, Hemlata. Thromboelastography as a novel viscoelastic method for hemostasis monitoring: Its methodology, applications, and constraints. Glob J Transfus Med. 2017;2:8. doi: 10.4103/GJTM.GJTM_4_17
Yuan W-H, Liu H-C, Zeng L-K, et al. [Change of Thrombelastography in Children’s DIC and Analysis of Its Sensitivity and Specificity for Diagnosis of DIC]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017;25:847–52. doi:10.7534/j.issn.1009-2137.2017.03.039.
Михельсон, В. А., В. А. Сидоров, and С. М. Степаненко. “Анестезия и интенсивная терапия в педиатрии.” М.:«Дель рус (2007).
Голуб И.Е., Сорокина Л.В. Избранные вопросы по общей анестезиологии (методические рекомендации для клинических ординаторов, с правом переиздания). – 2005.
Salem GI, Gamal NM, Talaat EA, et al. Clinical Impact of the ABO Blood Type in Patients with Rheumatic Diseases: Is there a Link to the ABO and Rhesus? Mediterr J Rheumatol. 2021;32:237. doi: 10.31138/mjr.32.3.237
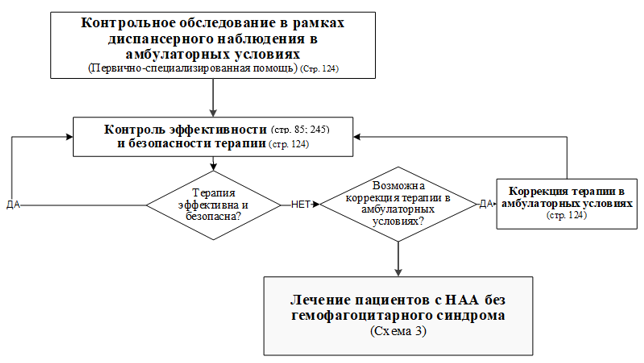
Клинический протокол МЗ РК. Неспецифический аортоартериит у детей. URL: https://diseases.medelement.com/disease/неспецифический-аортоартериит-у-детей-кп-рк-2023/17724.
Keser G, Aksu K, Direskeneli H. Takayasu arteritis: an update. Turk J Med Sci. 2018;48:681–97. doi: 10.3906/sag-1804-136
Eleftheriou D, Varnier G, Dolezalova P, et al. Takayasu arteritis in childhood: retrospective experience from a tertiary referral centre in the United Kingdom. Arthritis Res Ther. 2015;17:36. doi: 10.1186/s13075-015-0545-1
Kanzaki S, Kanda S. Coombs’ antibodies and rheumatoid factors in Takayasu’s arteritis. JAMA. 1985;254:232.
Zhang Y, Zhang D, Qu Y, et al. Anemia in patients with Takayasu arteritis: prevalence, clinical features, and treatment. J Geriatr Cardiol JGC. 2019;16:689–94. doi: 10.11909/j.issn.1671-5411.2019.09.003
Nishibukuro M, Tsutsumi N, Chiyotanda M, et al. Poststreptococcal reactive arthritis in Japan. J Infect Chemother Off J Jpn Soc Chemother. 2018;24:531–7. doi: 10.1016/j.jiac.2018.02.012
Windfuhr JP, Toepfner N, Steffen G, et al. Clinical practice guideline: tonsillitis I. Diagnostics and nonsurgical management. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2016;273:973–87. doi: 10.1007/s00405-015-3872-6
Tombetti E, DI Chio MC, Sartorelli S, et al. Procalcitonin in takayasu arteritis. J Rheumatol. 2014;41:1564–6. doi: 10.3899/jrheum.131340
Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46:10–67. doi: 10.1007/s00134-019-05878-6
Howell MD, Davis AM. Management of Sepsis and Septic Shock. JAMA. 2017;317:847. doi: 10.1001/jama.2017.0131
Limper M, de Kruif MD, Duits AJ, et al. The diagnostic role of Procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J Infect. 2010;60:409–16. doi: 10.1016/j.jinf.2010.03.016
Srivastava R, Phatak S, Yadav A, et al. HLA B27 typing in 511 children with juvenile idiopathic arthritis from India. Rheumatol Int. 2016;36:1407–11. doi: 10.1007/s00296-016-3529-9
Żuber Z, Turowska-Heydel D, Sobczyk M, et al. Prevalence of HLA-B27 antigen in patients with juvenile idiopathic arthritis. Reumatologia. 2015;53:125–30. doi: 10.5114/reum.2015.53133
Güzel Esen S, Armagan B, Atas N, et al. Increased incidence of spondyloarthropathies in patients with Takayasu arteritis: a systematic clinical survey. Joint Bone Spine. 2019;86:497–501. doi: 10.1016/j.jbspin.2019.01.020
Kalina T, Bakardjieva M, Blom M, et al. EuroFlow Standardized Approach to Diagnostic Immunopheneotyping of Severe PID in Newborns and Young Children. Front Immunol. 2020;11:371. doi: 10.3389/fimmu.2020.00371
Tavakol M, Jamee M, Azizi G, et al. Diagnostic Approach to the Patients with Suspected Primary Immunodeficiency. Endocr Metab Immune Disord Drug Targets. 2020;20:157–71. doi: 10.2174/1871530319666190828125316
Matsumoto K, Suzuki K, Yoshimoto K, et al. Significant association between clinical characteristics and changes in peripheral immuno-phenotype in large vessel vasculitis. Arthritis Res Ther. 2019;21:304. doi: 10.1186/s13075-019-2068-7
Румянцев А.Г., Масчан А.А., Щербина А.Ю. Федеральные клинические рекомендации по диагностике и лечению детей с тяжелой комбинированной иммунной недостаточностью. 2015.
Breda, L., Nozzi, M., De Sanctis, S., & Chiarelli, F. (2010). Laboratory Tests in the Diagnosis and Follow-Up of Pediatric Rheumatic Diseases: An Update. Seminars in Arthritis and Rheumatism, 40(1), 53–72. doi:10.1016/j.semarthrit.2008.12.
John RM, Kenney-Riley K. Care of the Child with a Possible Rheumatological Disorder. In: John RM, ed. Pediatric Diagnostic Labs for Primary Care: An Evidence-based Approach. Cham: Springer International Publishing 2022:461–86.
Ben-Chetrit E, Gattorno M, Gul A, et al. Consensus proposal for taxonomy and definition of the autoinflammatory diseases (AIDs): a Delphi study. Ann Rheum Dis. 2018;77:1558–65. doi: 10.1136/annrheumdis-2017-212515
Adrovic A, Sahin S, Barut K, et al. Familial Mediterranean fever and periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome: shared features and main differences. Rheumatol Int. 2019;39:29–36. doi: 10.1007/s00296-018-4105-2
Bourguiba R, Savey L, Aouba A, et al. Le syndrome de fièvre prolongée associée aux mutations du gène du récepteur au TNF de type 1 : un diagnostic différentiel de la fièvre méditerranéenne familiale à ne pas méconnaître chez les patients d’origine méditerranéenne. Rev Médecine Interne. 2021;42:459–64. doi: 10.1016/j.revmed.2020.08.007
Sag E, Bilginer Y, Ozen S. Autoinflammatory Diseases with Periodic Fevers. Curr Rheumatol Rep. 2017;19:41. doi: 10.1007/s11926-017-0670-8
Soon GS, Laxer RM. Approach to recurrent fever in childhood. Can Fam Physician Med Fam Can. 2017;63:756–62.
Hashkes PJ, Laxer RM, Simon A, editors. Textbook of Autoinflammation. 1st ed. 2019. Cham: Springer International Publishing : Imprint: Springer 2019.
Karadag O, Aksu K, Sahin A, et al. Assessment of latent tuberculosis infection in Takayasu arteritis with tuberculin skin test and Quantiferon-TB Gold test. Rheumatol Int. 2010;30:1483–7. doi: 10.1007/s00296-010-1444-z
Аксенова ВА, Барышников ЛА, Клевно НИ, et al. Новые возможности скрининга и диагностики различных проявлений туберкулезной инфекции у детей и подростков в России. Вопросы Современной Педиатрии. 2011;10:16–22.
García-Basteiro AL, DiNardo A, Saavedra B, et al. Point of care diagnostics for tuberculosis. Pulmonology. 2018;24:73–85. doi: 10.1016/j.rppnen.2017.12.002
Handa R, Upadhyaya S, Kapoor S, et al. Tuberculosis and biologics in rheumatology: A special situation. Int J Rheum Dis. 2017;20:1313–25. doi: 10.1111/1756-185X.13129
Cantini F, Nannini C, Niccoli L, et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev. 2015;14:503–9. doi: 10.1016/j.autrev.2015.01.011
Aksenova VA, Klevno NI, Kazakov AV, et al. Preventive Tuberculosis Services Reduces the Risk of Local Forms of Tuberculosis Development in Children on Immunosuppressive Therapy: Retrospective Cohort Study. Curr Pediatr. 2020;19:346–51. doi: 10.15690/vsp.v19i5.2210
Calzada-Hernández J, Anton J, Martín De Carpi J, et al. Dual latent tuberculosis screening with tuberculin skin tests and QuantiFERON-TB assays before TNF-α inhibitor initiation in children in Spain. Eur J Pediatr. 2022;182:307–17. doi: 10.1007/s00431-022-04640-3
Бармина НА, Барышникова ЛА, Шурыгин АА, et al. Скрининговое обследование детей и подростков III, IV и V групп здоровья с применением нового диагностического теста. Туберкулез И Болезни Легких. 2015;0:40–1.
Ferjani M, El Euch M, Boumediene M, et al. Tuberculosis and Takayasu arteritis: a case report. J Med Case Reports. 2023;17. doi: 10.1186/s13256-023-04037-2
Khemiri M, Douira W, Barsaoui S. Co-occurrence of Takayasu′s arteritis and tuberculosis: Report of a Tunisian pediatric case. Ann Pediatr Cardiol. 2016;9:75. doi: 10.4103/0974-2069.171398
Tian Y, Chen Y. Stroke in Takayasu arteritis with concomitant tuberculosis: an unusual pediatric case report. BMC Pediatr. 2022;22. doi: 10.1186/s12887-022-03125-4
Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13:546–9. doi: 10.1016/j.autrev.2014.01.005
Bentaleb I, Abdelghani KB, Rostom S, et al. Reactive Arthritis: Update. Curr Clin Microbiol Rep. 2020;1–9. doi: 10.1007/s40588-020-00152-6
Fujita M, Hatachi S, Yagita M. Acute Chlamydia pneumoniae infection in the pathogenesis of autoimmune diseases. Lupus. 2009;18:164–8. doi: 10.1177/0961203308096069
Rogozinski LE, Alverson BK, Biondi EA. Diagnosis and treatment of Mycoplasma pneumoniae in children. Minerva Pediatr. 2017;69:156–60. doi: 10.23736/S0026-4946.16.04866-0
Kwiatkowska B, Filipowicz‑Sosnowska A. Reactive arthritis. Pol Arch Intern Med. 2009;119:60–6. doi: 10.20452/pamw.606
Castillo RD, De La Pena W, Marzan KAB. Diagnosis and Management of Infectious Complications of Childhood Rheumatic Diseases. Curr Rheumatol Rep. 2013;15. doi: 10.1007/s11926-013-0322-6
Cuadros EN, Calzada-Hernández J, Clemente D, et al. Position statement of the Spanish Society of Pediatric Rheumatology on infection screening, prophylaxis, and vaccination of pediatric patients with rheumatic diseases and immunosuppressive therapies: Part 1 (screening). Eur J Pediatr. 2022;181:2343–54. doi: 10.1007/s00431-022-04418-7
Allen V, Longley N. Infections in immunosuppressed travellers with autoimmune inflammatory diseases—a narrative review and advice for clinical practice. Rheumatology. 2021;60:3969–76. doi: 10.1093/rheumatology/keab445
Whyte LA, Al-Araji RA, McLoughlin LM. Guidelines for the management of acute gastroenteritis in children in Europe. Arch Dis Child - Educ Pract Ed. 2015;100:308–12. doi: 10.1136/archdischild-2014-307253
Bockemühl J, Roggentin P. Enterale Yersiniosen. Klinische Bedeutung, Epidemiologie, Diagnostik und Prävention [Intestinal yersiniosis. Clinical importance, epidemiology, diagnosis, and prevention]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004 Jul;47(7):685-91
Houen G, Trier NH. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front Immunol. 2020;11:587380. doi: 10.3389/fimmu.2020.587380
Looker KJ, Magaret AS, May MT, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PloS One. 2015;10:e0140765. doi: 10.1371/journal.pone.0140765
McQuillan G, Kruszon-Moran D, Flagg EW, et al. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14-49: United States, 2015-2016. NCHS Data Brief. 2018;1–8.
Levin MJ, Weinberg A, Schmid DS. Herpes Simplex Virus and Varicella-Zoster Virus. Microbiol Spectr. 2016;4. doi: 10.1128/microbiolspec.DMIH2-0017-2015
Xu F, Lee FK, Morrow RA, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J Pediatr. 2007;151:374–7. doi: 10.1016/j.jpeds.2007.04.065
Shi T, Huang L, Tian J. Prevalence of Epstein-Barr Viral DNA among children at a single hospital in Suzhou, China. J Pediatr (Rio J). 2022;98:142–6. doi: 10.1016/j.jped.2021.05.006
Engelmann I, Petzold DR, Kosinska A, et al. Rapid quantitative PCR assays for the simultaneous detection of herpes simplex virus, varicella zoster virus, cytomegalovirus, Epstein-Barr virus, and human herpesvirus 6 DNA in blood and other clinical specimens. J Med Virol. 2008;80:467–77. doi: 10.1002/jmv.21095
for the Paediatric Rheumatology International Trials Organisation (PRINTO), Giancane G, Swart JF, et al. Opportunistic infections in immunosuppressed patients with juvenile idiopathic arthritis: analysis by the Pharmachild Safety Adjudication Committee. Arthritis Res Ther. 2020;22:71. doi: 10.1186/s13075-020-02167-2
Liu Z, Zhang P, Tang S, et al. Urine real-time polymerase chain reaction detection for children virus pneumonia with acute human cytomegalovirus infection. BMC Infect Dis. 2014;14:245. doi: 10.1186/1471-2334-14-245
A. Ross S, Novak Z, Pati S, et al. Overview of the Diagnosis of Cytomegalovirus Infection. Infect Disord - Drug Targets. 2011;11:466–74. doi: 10.2174/187152611797636703
Slots J, Slots H. Bacterial and viral pathogens in saliva: disease relationship and infectious risk. Periodontol 2000. 2011;55:48–69. doi: 10.1111/j.1600-0757.2010.00361.x
Strick LB, Wald A. Diagnostics for Herpes Simplex Virus: Is PCR the New Gold Standard? Mol Diagn Ther. 2006;10:17–28. doi: 10.1007/BF03256439
Vince A, Dusek D. Imunosupresija i virusne infekcije u reumatskim bolestima [Immunosupression and viral infections in rheumatic diseases]. Reumatizam. 2007;54(2):58-62. Croatian. PMID: 18351141.
Eisenstein EM, Wolf DG. Cytomegalovirus infection in pediatric rheumatic diseases: a review. Pediatr Rheumatol. 2010;8:17. doi: 10.1186/1546-0096-8-17
Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4:466–76. doi: 10.1016/S2468-1253(19)30042-1
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. doi: 10.1016/j.jhep.2017.03.021
Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4:477–87. doi: 10.1016/S2468-1253(19)30046-9
Mack CL, Adams D, Assis DN, et al. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatol Baltim Md. 2020;72:671–722. doi: 10.1002/hep.31065
Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. doi: 10.1016/j.jhep.2017.03.021
Harrison MJ, Brice N, Scott C. Clinical Features of HIV Arthropathy in Children: A Case Series and Literature Review. Front Immunol. 2021;12:677984. doi: 10.3389/fimmu.2021.677984
World Health Organization. WHO recommendations on the diagnosis of HIV infection in infants and children. World Health Organization, 2010.
Key recommendations. WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children. World Health Organization 2010.
McAuley JB. Congenital Toxoplasmosis. J Pediatr Infect Dis Soc. 2014;3 Suppl 1:S30-35. doi: 10.1093/jpids/piu077
McAuley JB. Toxoplasmosis in Children. Pediatr Infect Dis J. 2008;27:161–2. doi: 10.1097/INF.0b013e3181658abb
Villard O, Cimon B, L’Ollivier C, et al. Serological diagnosis of Toxoplasma gondii infection. Diagn Microbiol Infect Dis. 2016;84:22–33. doi: 10.1016/j.diagmicrobio.2015.09.009
Dong W, Zhong Q, Gu Y-L, et al. Is Toxoplasma gondii infection a concern in individuals with rheumatic diseases? Evidence from a case-control study based on serological diagnosis. Microb Pathog. 2023;182:106257. doi: 10.1016/j.micpath.2023.106257
Yagupsky P, Morata P, Colmenero JD. Laboratory Diagnosis of Human Brucellosis. Clin Microbiol Rev. 2019;33:e00073-19. doi: 10.1128/CMR.00073-19
Abdolsalehi M, Pourakbari B, Mahmoudi S, et al. Clinical and Epidemiologic Features of Visceral Leishmaniasis in Children: A 6-year Study from an Iranian Referral Hospital. Infect Disord Drug Targets. 2020;20:461–6. doi: 10.2174/1871526519666190613123217
Aronson NE, Joya CA. Cutaneous Leishmaniasis: Updates in Diagnosis and Management. Infect Dis Clin North Am. 2019;33:101–17. doi: 10.1016/j.idc.2018.10.004
Cascio A., Colomba C. Childhood Mediterranean visceral leishmaniasis //Le Infezioni in Medicina. – 2003. – Т. 11. – №. 1. – С. 5-10.
Moltó A, Mateo L, Lloveras N, et al. Visceral leishmaniasis and macrophagic activation syndrome in a patient with rheumatoid arthritis under treatment with adalimumab. Joint Bone Spine. 2010;77:271–3. doi: 10.1016/j.jbspin.2010.01.011
Kurizky PS, Marianelli FF, Cesetti MV, et al. A comprehensive systematic review of leishmaniasis in patients undergoing drug-induced immunosuppression for the treatment of dermatological, rheumatological and gastroenterological diseases. Rev Inst Med Trop São Paulo. 2020;62:e28. doi: 10.1590/s1678-9946202062028
Sood SK. Lyme Disease in Children. Infect Dis Clin North Am. 2015;29:281–94. doi: 10.1016/j.idc.2015.02.011
Steffen, Hirsch. Diagnostik der Lyme-Borreliose. Ther Umsch. 2005;62:737–44. doi: 10.1024/0040-5930.62.11.737
Robinson JL, Lee BE, Kothapalli S, et al. Use of Throat Swab or Saliva Specimens for Detection of Respiratory Viruses in Children. Clin Infect Dis. 2008;46:e61–4. doi: 10.1086/529386
Fotis L, Kourti A, Prountzos S, et al. Takayasu arteritis in an adolescent with Crohn’s disease. Rheumatol Int. 2022;42:563–70. doi: 10.1007/s00296-021-04869-5
Balamtekin N, Gürakan F, Ozen S, et al. Ulcerative colitis associated with Takayasu’s arteritis in a child. Acta Paediatr Oslo Nor 1992. 2009;98:1368–71. doi: 10.1111/j.1651-2227.2009.01330.x
Guarino AD, Testa A, Mormile I, et al. Crohn’s disease and Takayasu’s arteritis: are they associated? Eur Rev Med Pharmacol Sci. 2021;25:1472–84. doi: 10.26355/eurrev_202102_24855
Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care—An Evidence-based Guideline From European Crohnʼs and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:257–91. doi: 10.1097/MPG.0000000000002035
Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. Published Online First: 5 June 2014. doi: 10.1016/j.crohns.2014.04.005
Ferrara G, Pastore S, Sancin L, et al. Fecal Calprotectin to Detect Inflammatory Bowel Disease in Juvenile Idiopathic Arthritis. J Rheumatol. 2018;45:1418–21. doi: 10.3899/jrheum.171200
Chien Y-L, Huang F-L, Huang C-M, et al. Clinical approach to fever of unknown origin in children. J Microbiol Immunol Infect. 2017;50:893–8. doi: 10.1016/j.jmii.2015.08.007
Ahmadinejad Z, Mansori S, Ziaee V, et al. Periodic Fever: a review on clinical, management and guideline for Iranian patients - part I. Iran J Pediatr 2014;24:1–13.
Ören H, Cingöz I, Duman M, et al. Disseminated intravascular coagulation in pediatric patients: Clinical and Laboratory Features and Prognostic Factors Influencing the Survival. Pediatr Hematol Oncol. 2005;22:679–88. doi: 10.1080/08880010500278749
Li J, Li H, Sun F, et al. Clinical Characteristics of Heart Involvement in Chinese Patients with Takayasu Arteritis. J Rheumatol. 2017;44:1867–74. doi: 10.3899/jrheum.161514
An X, Han Y, Zhang B, et al. Takayasu arteritis presented with acute heart failure: case report and review of literature. ESC Heart Fail. 2017;4:649–54. doi: 10.1002/ehf2.12174
Alexeeva EI. Juvenile idiopathic arthritis: clinical picture, diagnosis, treatment. Curr Pediatr. 2015;14:78–94. doi: 10.15690/vsp.v14i1.1266
Koca B, Sahin S, Adrovic A, et al. Cardiac involvement in juvenile idiopathic arthritis. Rheumatol Int. 2017;37:137–42. doi: 10.1007/s00296-016-3534-z
Textbook of Pediatric Rheumatology. Elsevier 2016.
Cavoli GL, Bono L, Finazzo F, et al. The Magnetic Resonance Imaging in the Takayasu’s Arteritis. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2021;32:265–7. doi: 10.4103/1319-2442.318537
Sivakova O, Chikhladze N, Rogoza A, et al. The importance of 24-hour ambulatory blood pressure monitoring in takayasu arteritis.: PP.17.69. J Hypertens. 2011;29:e310. doi: 10.1097/00004872-201106001-00893
Haaversen ACB, Diamantopoulos AP. Ultrasound in Large Vessel Vasculitis. In: Akram Q, Basu S, eds. Ultrasound in Rheumatology: A Practical Guide for Diagnosis. Cham: Springer International Publishing 2021:207–35.
Germanò G, Monti S, Ponte C, et al. The role of ultrasound in the diagnosis and follow-up of large-vessel vasculitis: an update. Clin Exp Rheumatol. 2017;35 Suppl 103:194–8.
Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30. doi: 10.1136/annrheumdis-2019-215672
Barra L, Kanji T, Malette J, et al. Imaging modalities for the diagnosis and disease activity assessment of Takayasu’s arteritis: A systematic review and meta-analysis. Autoimmun Rev. 2018;17:175–87. doi: 10.1016/j.autrev.2017.11.021
Soliman M, Laxer R, Manson D, et al. Imaging of systemic vasculitis in childhood. Pediatr Radiol. 2015;45:1110–25. doi: 10.1007/s00247-015-3339-3
Gaballah M, Goldfisher R, Amodio JB. The Utility of MRI in the Diagnosis of Takayasu Arteritis. Case Rep Pediatr. 2017;2017:7976165. doi: 10.1155/2017/7976165
Russo RAG, Katsicas MM. Takayasu Arteritis. Front Pediatr. 2018;6:265. doi: 10.3389/fped.2018.00265
Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636–43. doi: 10.1136/annrheumdis-2017-212649
Schäfer VS, Jin L, Schmidt WA. Imaging for Diagnosis, Monitoring, and Outcome Prediction of Large Vessel Vasculitides. Curr Rheumatol Rep. 2020;22:76. doi: 10.1007/s11926-020-00955-y
Barra L, Kanji T, Malette J, et al. Imaging modalities for the diagnosis and disease activity assessment of Takayasu’s arteritis: A systematic review and meta-analysis. Autoimmun Rev. 2018;17:175–87. doi: 10.1016/j.autrev.2017.11.021
Clemente G, Pereira RMR, Aikawa N, et al. Is positron emission tomography/magnetic resonance imaging a reliable tool for detecting vascular activity in treated childhood-onset Takayasu’s arteritis? A multicentre study. Rheumatol Oxf Engl. 2022;61:554–62. doi: 10.1093/rheumatology/keab255
Лукьянчик Ю.Д., Ушакова С.А., Чернышева Т.В., et al. Трудности диагностики неспецифического аортоартериита у подростка. Университетская Медицина Урала. 2019;5:84–7.
Sönmez HE, Demir F, Özdel S, et al. Neuroimaging of Children With Takayasu Arteritis. J Child Neurol. 2021;36:642–7. doi: 10.1177/0883073821991287
Zaki SA, Chavan V, Shanbag P. Unusual presentation of Takayasu’s arteritis as posterior reversible encephalopathy syndrome. Ann Indian Acad Neurol. 2011;14:214–6. doi: 10.4103/0972-2327.85900
Weiss PF, Corao DA, Pollock AN, et al. Takayasu arteritis presenting as cerebral aneurysms in an 18 month old: A case report. Pediatr Rheumatol Online J. 2008;6:4. doi: 10.1186/1546-0096-6-4
Hernandez J, Polo R, Alvarez J, et al. [A “lupus-like” syndrome as the form of presentation of pulmonary adenocarcinoma]. An Med Interna Madr Spain 1984. 2000;17:558–9.
Allen-Rhoades W, Whittle SB, Rainusso N. Pediatric Solid Tumors in Children and Adolescents: An Overview. Pediatr Rev. 2018;39:444–53. doi: 10.1542/pir.2017-0268
Fonseca MB, Gomes FHR, Valera ET, et al. Signs and symptoms of rheumatic diseases as first manifestation of pediatric cancer: diagnosis and prognosis implications. Rev Bras Reumatol Engl Ed. 2017;57:330–7. doi: 10.1016/j.rbre.2017.01.007
Alias A, Rodriguez EJ, Bateman HE, et al. Rheumatology and oncology: an updated review of rheumatic manifestations of malignancy and anti-neoplastictherapy. Bull NYU Hosp Jt Dis. 2012;70:109–14.
Hall S, Nelson AM. Takayasu’s arteritis and juvenile rheumatoid arthritis. J Rheumatol. 1986;13:431–3.
Sukharomana M, Viravan S, Piyaphanee N, et al. Takayasu arteritis with an initial presentation of chronic monoarthritis mimicking oligoarticular juvenile idiopathic arthritis. Pediatr Rep. 2018;10:7648. doi: 10.4081/pr.2018.7648
Clemente G, Silva CA, Sacchetti SB, et al. Takayasu arteritis in childhood: misdiagnoses at disease onset and associated diseases. Rheumatol Int. 2018;38:1089–94. doi: 10.1007/s00296-018-4030-4
Sy A, Khalidi N, Dehghan N, et al. Vasculitis in patients with inflammatory bowel diseases: A study of 32 patients and systematic review of the literature. Semin Arthritis Rheum. 2016;45:475–82. doi: 10.1016/j.semarthrit.2015.07.006
Gong EJ, Kim DH, Chun JH, et al. Endoscopic Findings of Upper Gastrointestinal Involvement in Primary Vasculitis. Gut Liver. 2016;10:542–8. doi: 10.5009/gnl15198
Trapani S, Rubino C, Indolfi G. Gastrointestinal involvement in childhood vasculitides. Acta Paediatr Oslo Nor 1992. 2020;109:2226–36. doi: 10.1111/apa.15381
Sierra D, Wood M, Kolli S, et al. Pediatric Gastritis, Gastropathy, and Peptic Ulcer Disease. Pediatr Rev. 2018;39:542–9. doi: 10.1542/pir.2017-0234
Jones NL, Koletzko S, Goodman K, et al. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter pylori in Children and Adolescents (Update 2016). J Pediatr Gastroenterol Nutr. 2017;64:991–1003. doi: 10.1097/MPG.0000000000001594
Kotilea K, Cadranel S, Salame A, et al. Efficacy and safety of bismuth‐based quadruple therapy for Helicobacter pylori eradication in children. Helicobacter. 2021;26:e12825. doi: 10.1111/hel.12825
Бельмер С.В., Корниенко Е.А., Волынец Г.В., Гурова М.М., Звягин А.А., Камалова А.А., Луппова Н.Е., Нижевич А.А., Новикова В.П., Печкуров Д.В., Приворотский В.Ф., Сатаев В.У., Тяжева А.А., Файзуллина Р.А., Хавкин А.И. Диагностика и лечение хеликобактерной инфекции у детей. Экспериментальная и клиническая гастроэнтерология. 2021;(9):119-127. https://doi.org/10.31146/1682-8658-ecg-193-9-119-127.
Evirgen Şahin G, Özdel S, Özbay Hoşnut F, et al. Takayasu’s arteritis diagnosed in a patient with Crohn’s disease: An unpredicted correlation. Arch Rheumatol. 2021;36:135–7. doi: 10.46497/ArchRheumatol.2021.8021
Passo MH, Fitzgerald JF, Brandt KD. Arthritis associated with inflammatory bowel disease in children: Relationship of joint disease to activity and severity of bowel lesion. Dig Dis Sci. 1986;31:492–7. doi: 10.1007/BF01320313
Biswas S. et al. IBD-Associated Arthritis Misdiagnosed as Juvenile Idiopathic Arthritis: A Case Report and Literature Review: 2091 //Official journal of the American College of Gastroenterology| ACG. – 2018. – Т. 113. – С. S1188-S1189.
Nasser M, Cottin V. The Respiratory System in Autoimmune Vascular Diseases. Respir Int Rev Thorac Dis. 2018;96:12–28. doi: 10.1159/000486899
Quezada A, Ramos S, Garcia M, et al. Lung involvement in rheumatologic diseases in children. Allergol Immunopathol (Madr). 2012;40:88–91. doi: 10.1016/j.aller.2011.02.009
Belvis Jiménez M, Maldonado Pérez B, Caunedo Álvarez A. Abdominal Pain in a Patient With Takayasu Arteritis. Am J Gastroenterol. 2020;115:950–1. doi: 10.14309/ajg.0000000000000612
Sasae Y, Morita Y, Sakuta T, et al. Abdominal pain as the initial presentation of Takayasu arteritis. Mod Rheumatol. 2008;18:496–8. doi: 10.1007/s10165-008-0075-7
Zhang Y, Fan P, Luo F, et al. Tuberculosis in Takayasu arteritis: a retrospective study in 1105 Chinese patients. J Geriatr Cardiol JGC. 2019;16:648–55. doi: 10.11909/j.issn.1671-5411.2019.08.003
Eutsler EP, Khanna G. Whole-body magnetic resonance imaging in children: technique and clinical applications. Pediatr Radiol. 2016;46:858–72. doi: 10.1007/s00247-016-3586-y
Yuce Inel T, Gulcu A, Karakas A, et al. Coexistence of Takayasu arteritis and chronic myeloid leukemia: Coincidental or paraneoplastic phenomenon? Int J Rheum Dis. 2021;24:1213–6. doi: 10.1111/1756-185X.14186
Chandratilleke D, Anantharajah A, Vicaretti M, et al. Migratory large vessel vasculitis preceding acute myeloid leukemia: a case report. J Med Case Reports. 2017;11:71. doi: 10.1186/s13256-017-1239-x
English SW, Klaas JP. Neurologic complications of diseases of the aorta. Handb Clin Neurol. 2021;177:221–39. doi: 10.1016/B978-0-12-819814-8.00028-7
Kim H-A, Kim J-H, Won J-H, et al. An unusual clinical manifestation of Takayasu’s arteritis: spinal cord compression. Joint Bone Spine. 2009;76:209–12. doi: 10.1016/j.jbspin.2008.09.007
Park JK, Choi IA, Lee EY, et al. Incidence of malignancy in Takayasu arteritis in Korea. Rheumatol Int. 2014;34:517–21. doi: 10.1007/s00296-013-2887-9
Zucker EJ, Lee EY, Restrepo R, et al. Hip disorders in children. AJR Am J Roentgenol. 2013;201:W776-796. doi: 10.2214/AJR.13.10623
Loctin A, Bailly F, Laroche D, et al. Clinical interest of bone marrow aspiration in rheumatology: a practice-based observational study of 257 bone marrow aspirations. Clin Rheumatol. 2013;32:115–21. doi: 10.1007/s10067-012-2097-y
Grebenyuk V. et al. Fever of unknown origin: case reports from routine clinical practice and a review //Klinicka Mikrobiologie a Infekcni Lekarstvi. – 2021. – Т. 27. – №. 4. – С. 148-157.
Захарова И.Н., Османов И.М., Творогова Т.М., Горяйнова А.Н., Дмитриева Ю.А., Воробьева А.С., Короид Н.В. Длительная лихорадка у ребенка: в чем причина, как обследовать, лечить или не лечить? Медицинский совет. 2020;(10):151–162. doi: 10.21518/2079-701X-2020-10-151-162.
Trapani S, Grisolia F, Simonini G, et al. Incidence of occult cancer in children presenting with musculoskeletal symptoms: a 10-year survey in a pediatric rheumatology unit. Semin Arthritis Rheum. 2000;29:348–59. doi: 10.1053/sarh.2000.5752
Morrissey NJ, Goldman J, Fallon JT, et al. Endovascular aortic biopsy in the diagnosis of takayasu arteritis. J Endovasc Ther Off J Int Soc Endovasc Spec. 2003;10:136–40. doi: 10.1177/152660280301000126
Restrepo CS, Betancourt SL, Martinez-Jimenez S, et al. Aortic tumors. Semin Ultrasound CT MR. 2012;33:265–72. doi: 10.1053/j.sult.2011.10.001
Li Q. et al. Quantifying the contribution of 18F-FDG PET to the diagnostic assessment of pediatric patients with fever of unknown origin: a systematic review and meta-analysis //Pediatric Radiology. – 2022. – С. 1-12.
Ćwikła JB. New imaging techniques in reumathology: MRI, scintigraphy and PET. Pol J Radiol. 2013;78:48–56. doi: 10.12659/PJR.889138
Colamussi P, Prandini N, Cittanti C, et al. Scintigraphy in rheumatic diseases. Best Pract Res Clin Rheumatol. 2004;18:909–26. doi: 10.1016/j.berh.2004.07.003
Clemente D, Cuadros EN, Lovillo MC, et al. Position statement on infection screening, prophylaxis, and vaccination of pediatric patients with rheumatic diseases and immunosuppressive therapies, part 3: precautions in situations of surgery, fever, and opportunistic infections. Eur J Pediatr. 2023;183:915–27. doi: 10.1007/s00431-023-05295-4
Bilal B, Oksuz G, Urfalioglu A, et al. Low Dose Spinal Anaesthesia for a Pediatric Patient with Takayasu and #8217;s Artheritis Undergoing Orthopaedic Surgery: a case Report. Med Sci Int Med J. 2016;5:76. doi: 10.5455/medscience.2016.05.8408
Medscape.Takayasu Arteritis Differential Diagnoses URL: https://emedicine.medscape.com/article/332378-differential?form=fpf.
Akar S, Dogan E, Goktay Y, et al. Nasal septal perforation in a patient with Takayasu’s arteritis; a rare association. Intern Med Tokyo Jpn. 2009;48:1551–4. doi: 10.2169/internalmedicine.48.2198
Anguita R, Nazar C, Kobus R, et al. Bilateral ocular ischemic syndrome as a manifestation of Takayasu arteritis in children. Can J Ophthalmol J Can Ophtalmol. 2019;54:e105–8. doi: 10.1016/j.jcjo.2018.08.018
McDonald MA, Ojaimi E, Favilla I. Anterior uveitis in a child with Takayasu’s arteritis. Clin Experiment Ophthalmol. 2004;32:336–9. doi: 10.1111/j.1442-9071.2004.00828.x
Jales-Neto LH, Levy-Neto M, Bonfa E, et al. Juvenile-onset Takayasu arteritis: peculiar vascular involvement and more refractory disease. Scand J Rheumatol. 2010;39:506–10. doi: 10.3109/03009741003742730
Duque C, Silva RC, Santos-Pinto L. Takayasu’s arteritis: what should the dentist know? Int J Paediatr Dent. 2005;15:113–7. doi: 10.1111/j.1365-263X.2005.00598.x
Radwan‐Oczko M, Duś‐Ilnicka I, Richards P, et al. Rheumatoid arthritis patients’ oral health and disease activity. Int J Rheum Dis. 2019;22:1538–43. doi: 10.1111/1756-185X.13590
Benjaminsen E, Reigstad A, Cengija V, et al. Stroke as the Sole Manifestation of Takayasu Arteritis in a 15-Year-Old Boy with Latent Tuberculosis. Case Rep Neurol Med. 2016;2016:8736248. doi: 10.1155/2016/8736248
Brunner J, Armstrong D, Feldman BM, et al. Childhood stroke as the presentation of Takayasu’s arteritis: diagnostic delay can cause catastrophic complications. J Rheumatol. 2008;35:1228–30.
Clemente G, Hilário MO, Len C, et al. Brazilian multicenter study of 71 patients with juvenile-onset Takayasu’s arteritis: clinical and angiographic features. Rev Bras Reumatol. 2016;56:145–51. doi: 10.1016/j.rbre.2016.01.004
Li-xin Z, Jun N, Shan G, et al. Neurological manifestations of Takayasu arteritis. Chin Med Sci J Chung-Kuo Hsueh Ko Hsueh Tsa Chih. 2011;26:227–30. doi: 10.1016/s1001-9294(12)60005-4
Lvovich S, Goldsmith DP. Neurological Complications of Rheumatic Disease. Semin Pediatr Neurol. 2017;24:54–9. doi: 10.1016/j.spen.2016.12.007
Shiari R. Neurologic manifestations of childhood rheumatic diseases. Iran J Child Neurol. 2012;6:1–7.
Vettiyil G, Punnen A, Kumar S. An Unusual Association of Chronic Recurrent Multifocal Osteomyelitis, Pyoderma Gangrenosum, and Takayasu Arteritis. J Rheumatol. 2017;44:127–8. doi: 10.3899/jrheum.160491
Misra DP, Aggarwal A, Lawrence A, et al. Pediatric-onset Takayasu’s arteritis: clinical features and short-term outcome. Rheumatol Int. 2015;35:1701–6. doi: 10.1007/s00296-015-3272-7
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter. 2013;3:1–150.
Li Cavoli G, Mulè G, Vallone MG, et al. Takayasu’s disease effects on the kidneys: current perspectives. Int J Nephrol Renov Dis. 2018;11:225–33. doi: 10.2147/IJNRD.S146355
Gutiérrez-González LA. Rheumatologic emergencies. Clin Rheumatol. 2015;34:2011–9. doi: 10.1007/s10067-015-2994-y
Yoshida M, Yamamoto T, Shiiba S, et al. Anesthetic Management of a Patient With Takayasu Arteritis. Anesth Prog. 2016;63:31–3. doi: 10.2344/14-00006R1.1
Röher K, Trieschmann U, Leister N. Anästhesie und Analgosedierung für diagnostische Eingriffe bei Kindern. AINS - Anästhesiol · Intensivmed · Notfallmedizin · Schmerzther. 2023;58:409–20. doi: 10.1055/a-1925-7009
Dumas G, Arabi YM, Bartz R, et al. Diagnosis and management of autoimmune diseases in the ICU. Intensive Care Med. 2024;50:17–35. doi: 10.1007/s00134-023-07266-7
Janssen NM, Karnad DR, Guntupalli KK. Rheumatologic diseases in the intensive care unit: epidemiology, clinical approach, management, and outcome. Crit Care Clin. 2002;18:729–48. doi: 10.1016/S0749-0704(02)00025-8
Hsieh L-F, Mao H-F, Lu C-C, et al. 31 - Rheumatologic Rehabilitation. In: Cifu DX, ed. Braddom’s Physical Medicine and Rehabilitation (Sixth Edition). Philadelphia: Elsevier 2021:606-626.e1.
Cohen EM, Morley-Fletcher A, Mehta DH, et al. A systematic review of psychosocial therapies for children with rheumatic diseases. Pediatr Rheumatol Online J. 2017;15:6. doi: 10.1186/s12969-016-0133-1
Ferreira E, Gramasco H, Iglesias S. Pediatric rheumatology and palliative care for children: a most relevant match. Residência Pediátrica. 2019;9:189–92. doi: 10.25060/residpediatr-2019.v9n2-21
Mackay M, Verstegen R, Hawley D, et al. 5. Paediatric Takayasu arteritis. Rheumatol Adv Pract. 2018;2:rky031.004. doi: 10.1093/rap/rky031.004
Frye WS, Milojevic D. The Role of Psychology in Pediatric Rheumatic Diseases. Pediatr Clin North Am. 2022;69:965–74. doi: 10.1016/j.pcl.2022.05.009
Chyzheuskaya I. et al. THU0508 Psychological features of children with rheumatic diseases. – 2017.
Ferro MA, Boyle MH. Self-concept among youth with a chronic illness: A meta-analytic review. Health Psychol. 2013;32:839–48. doi: 10.1037/a0031861
Cousino MK, Hazen RA. Parenting Stress Among Caregivers of Children With Chronic Illness: A Systematic Review. J Pediatr Psychol. 2013;38:809–28. doi: 10.1093/jpepsy/jst049
Knafl K, Leeman J, Havill NL, et al. The Contribution of Parent and Family Variables to the Well-Being of Youth With Arthritis. J Fam Nurs. 2015;21:579–616. doi: 10.1177/1074840715601475
Henderson LA, Cron RQ. Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Childhood Inflammatory Disorders: Diagnosis and Management. Paediatr Drugs. 2020;22:29–44. doi: 10.1007/s40272-019-00367-1
Ravelli A, Davì S, Minoia F, et al. Macrophage Activation Syndrome. Hematol Oncol Clin North Am. 2015;29:927–41. doi: 10.1016/j.hoc.2015.06.010
Shakoory B, Geerlinks A, Wilejto M, et al. The 2022 EULAR/ACR points to consider at the early stages of diagnosis and management of suspected haemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS). Ann Rheum Dis. 2023;82:1271–85. doi: 10.1136/ard-2023-224123
Hines MR, von Bahr Greenwood T, Beutel G, et al. Consensus-Based Guidelines for the Recognition, Diagnosis, and Management of Hemophagocytic Lymphohistiocytosis in Critically Ill Children and Adults. Crit Care Med. 2022;50:860–72. doi: 10.1097/CCM.0000000000005361
Alongi A, Naddei R, De Miglio L, et al. Macrophage activation syndrome in pediatrics. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2020;31 Suppl 24:13–5. doi: 10.1111/pai.13158
Lehmberg K, Pink I, Eulenburg C, et al. Differentiating Macrophage Activation Syndrome in Systemic Juvenile Idiopathic Arthritis from Other Forms of Hemophagocytic Lymphohistiocytosis. J Pediatr. 2013;162:1245–51. doi: 10.1016/j.jpeds.2012.11.081
Chen T-Y, Hsu M-H, Kuo H-C, et al. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. 2021;120:172–9. doi: 10.1016/j.jfma.2020.03.025
Rigante D, Emmi G, Fastiggi M, et al. Macrophage activation syndrome in the course of monogenic autoinflammatory disorders. Clin Rheumatol. 2015;34:1333–9. doi: 10.1007/s10067-015-2923-0
Valade S, Mariotte E, Azoulay E. Coagulation Disorders in Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome. Crit Care Clin. 2020;36:415–26. doi: 10.1016/j.ccc.2019.12.004
Schapkaitz E, Sherman GG, Jacobson BF, et al. Paediatric anticoagulation guidelines. S Afr Med J. 2012;102:171. doi: 10.7196/SAMJ.5471
Manco-Johnson MJ. How I treat venous thrombosis in children. Blood. 2006;107:21–9. doi: 10.1182/blood-2004-11-4211
Tolbert J, Carpenter SL. Common Acquired Causes of Thrombosis in Children. Curr Probl Pediatr Adolesc Health Care. 2013;43:169–77. doi: 10.1016/j.cppeds.2013.05.005
Valade S, Azoulay E, Galicier L, et al. Coagulation Disorders and Bleedings in Critically Ill Patients With Hemophagocytic Lymphohistiocytosis. Medicine (Baltimore). 2015;94:e1692. doi: 10.1097/MD.0000000000001692
Xiang, L., Qian, J., Zhang, J., Ren, H., HU, X., LI, B., ... & NING, B. (2017). Clinical study of thromboelastography for assessment of coagulation disorders in children with sepsis. Chinese Journal of Emergency Medicine, 1284-1289.
Iakunina LN, Plakhuta TG, Tsymbal IN. [State of hemostasis in hemorrhagic vasculitis in children]. Pediatriia. 1992;16–20.
Law C, Raffini L. A Guide to the Use of Anticoagulant Drugs in Children. Pediatr Drugs. 2015;17:105–14. doi: 10.1007/s40272-015-0120-x
Islam MI, Talukder MK, Islam MM, et al. Macrophage Activation Syndrome in Paediatric Rheumatic Diseases. Mymensingh Med J MMJ. 2017;26:356–63.
Avčin T, Tse SML, Schneider R, et al. Macrophage activation syndrome as the presenting manifestation of rheumatic diseases in childhood. J Pediatr. 2006;148:683–6. doi: 10.1016/j.jpeds.2005.12.070
Bagri NK, Gupta L, Sen ES, et al. Macrophage Activation Syndrome in Children: Diagnosis and Management. Indian Pediatr. 2021;58:1155–61. doi: 10.1007/s13312-021-2399-8
Abolghasemi H, Shahverdi E, Niknam R, et al. Macrophage Activation Syndrome as the First Presentation of Juvenile Idiopathic Arthritis. IJBC. 2017;9:93–6.
Ansuini V, Rigante D, Esposito S. Debate around infection-dependent hemophagocytic syndrome in paediatrics. BMC Infect Dis. 2013;13:15. doi: 10.1186/1471-2334-13-15
D’Errico MM, Cuoco F, Biancardi C, et al. YIM-P58. Macrophage activation syndrome: the role of infectious triggers. Pediatr Rheumatol. 2014;12:Y5, 1546-0096-12-S1-Y5. doi: 10.1186/1546-0096-12-S1-Y5
Eloseily EM, Weiser P, Crayne CB, et al. Benefit of Anakinra in Treating Pediatric Secondary Hemophagocytic Lymphohistiocytosis. Arthritis Rheumatol. 2020;72:326–34. doi: 10.1002/art.41103
Eloseily EM, Cron RQ. Macrophage Activation Syndrome. In: Ragab G, Atkinson TP, Stoll ML, eds. The Microbiome in Rheumatic Diseases and Infection. Cham: Springer International Publishing 2018:151–82.
Long S. S., Pickering L. K., Prober C. G. Principles and practice of pediatric infectious diseases. – 2023.
Xu D, Li S, Chen Z, et al. Detection of Mycoplasma pneumoniae in different respiratory specimens. Eur J Pediatr. 2011;170:851–8. doi: 10.1007/s00431-010-1360-y
Copete AR, Vera C, Herrera M, et al. Mycoplasma pneumoniae in Children With and Without Community-acquired Pneumonia. What do PCR and Serology Say? Pediatr Infect Dis J. 2020;39:e104–8. doi: 10.1097/INF.0000000000002636
Waris ME, Toikka P, Saarinen T, et al. Diagnosis of Mycoplasma pneumoniae Pneumonia in Children. J Clin Microbiol. 1998;36:3155–9. doi: 10.1128/JCM.36.11.3155-3159.1998
Department of Microbiology, Maulana Azad Medical College, India, Kumar S, Kumar S, et al. Mycoplasma Pneumoniae as a Causative Agent of Community-Acquired Lower Respiratory Tract Infections in Children. Ann Pediatr Child Health. 2023;11:1–4. doi: 10.47739/2373-9312.pediatrics.1325
Banko A, Cirkovic A, Jeremic I, et al. Uncovering the Role of Epstein–Barr Virus Infection Markers for Remission in Rheumatoid Arthritis. Biomedicines. 2023;11:2375. doi: 10.3390/biomedicines11092375
Blanche S, Caniglia M, Fischer A, et al. Epstein-Barr Virus-Associated Hemophagocytic Syndrome: Clinical Presentation and Treatment. Pediatr Hematol Oncol. 1989;6:233–5. doi: 10.3109/08880018909034292
Nowalk A, Green M. Epstein-Barr Virus. Microbiol Spectr. 2016;4:4.3.47. doi: 10.1128/microbiolspec.DMIH2-0011-2015
Brisse E, Matthys P, Wouters CH. Understanding the spectrum of haemophagocytic lymphohistiocytosis: update on diagnostic challenges and therapeutic options. Br J Haematol. 2016;174:175–87. doi: 10.1111/bjh.14144
Levin MJ, Weinberg A, Schmid DS. Herpes Simplex Virus and Varicella-Zoster Virus. Microbiol Spectr. 2016;4:4.3.49. doi: 10.1128/microbiolspec.DMIH2-0017-2015
Nadimpalli S, Foca M, Satwani P, et al. Diagnostic yield of bronchoalveolar lavage in immunocompromised children with malignant and non-malignant disorders: BAL in Immunocompromised Children. Pediatr Pulmonol. 2017;52:820–6. doi: 10.1002/ppul.23644
Ers. Bronchoalveolar lavage in children. Eur Respir J. 2000;15:217–31. doi: 10.1183/09031936.00.15121700
Amanati A, Karimi A, Fahimzad A, et al. Prevalence of Human Herpes Viruses in Bronchoalveolar Lavage of Critically Ill Children Undergoing Mechanical Ventilation at a Pediatric Intensive Care Unit. Arch Pediatr Infect Dis. 2018;6. doi: 10.5812/pedinfect.12685
Wong JCP, Hon KLE, Leung KKY, et al. Diagnostic Yield of Bronchoalveolar Lavage in Immunocompromised Children. J Trop Pediatr. 2021;67:fmaa131. doi: 10.1093/tropej/fmaa131
Eroglu-Ertugrul NG, Yalcin E, Oguz B, et al. The value of flexible bronchoscopy in pulmonary infections of immunosuppressed children. Clin Respir J. 2020;14:78–84. doi: 10.1111/crj.13103
Gonski K, Cohn R, Widger J, et al. Utility of bronchoscopy in immunocompromised paediatric patients: Systematic review. Paediatr Respir Rev. 2020;34:24–34. doi: 10.1016/j.prrv.2020.02.003
Özkoç S, Bayram Delibaş S. [Investigation of Pneumocystis jirovecii pneumonia and colonization in iatrogenically immunosuppressed and immunocompetent patients]. Mikrobiyol Bul. 2015;49:221–30. doi: 10.5578/mb.9344
Lachant DJ, Croft DP, McGrane Minton H, et al. The clinical impact of pneumocystis and viral PCR testing on bronchoalveolar lavage in immunosuppressed patients. Respir Med. 2018;145:35–40. doi: 10.1016/j.rmed.2018.10.021
Stephan JL. Reactive haemophagocytic syndrome in children with inflammatory disorders. A retrospective study of 24 patients. Rheumatology. 2001;40:1285–92. doi: 10.1093/rheumatology/40.11.1285
Varadaraju S, Khandelwal P, Sankar J, et al. Multiple opportunistic infection-associated hemophagocytic lymphohistiocytosis in nephrotic syndrome: A case report. J Pediatr Crit Care. 2021;8:295. doi: 10.4103/jpcc.jpcc_64_21
Weyant RB, Kabbani D, Doucette K, et al. Pneumocystis jirovecii: a review with a focus on prevention and treatment. Expert Opin Pharmacother. 2021;22:1579–92. doi: 10.1080/14656566.2021.1915989
Morris A, Norris KA. Colonization by Pneumocystis jirovecii and Its Role in Disease. Clin Microbiol Rev. 2012;25:297–317. doi: 10.1128/CMR.00013-12
White PL, Price JS, Backx M. Pneumocystis jirovecii Pneumonia: Epidemiology, Clinical Manifestation and Diagnosis. Curr Fungal Infect Rep. 2019;13:260–73. doi: 10.1007/s12281-019-00349-3
Schüller M, Macků M, Fráňová J. Pneumocystis pneumonia in a child with sojia. Pediatr Rheumatol. 2014;12:P225, 1546-0096-12-S1-P225. doi: 10.1186/1546-0096-12-S1-P225
Saper V. E. et al. Emergent high fatality lung disease in systemic juvenile arthritis //Annals of the rheumatic diseases. – 2019. – Т. 78. – №. 12. – С. 1722-1731.
Rayment JH, Narang I. Pulmonary Aspergillosis in a Previously Healthy 13-Year-Old Boy. Can Respir J. 2016;2016:4575942. doi: 10.1155/2016/4575942
de Mol M, de Jongste JC, van Westreenen M, et al. Diagnosis of invasive pulmonary aspergillosis in children with bronchoalveolar lavage galactomannan: BAL Galactomannan Aspergillosis Children. Pediatr Pulmonol. 2013;48:789–96. doi: 10.1002/ppul.22670
Signoff JK, Fitzgerald JC, Teachey DT, et al. Hypofibrinogenemia Is Associated With Poor Outcome and Secondary Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome in Pediatric Severe Sepsis*: Pediatr Crit Care Med. 2018;19:397–405. doi: 10.1097/PCC.0000000000001507
Machowicz R, Janka G, Wiktor-Jedrzejczak W. Similar but not the same: Differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol. 2017;114:1–12. doi: 10.1016/j.critrevonc.2017.03.023
Zhao M, Guan Y, Lin J, et al. Acute kidney injury in critical care: complications of hemophagocytic lymphohistiocytosis. Front Immunol. 2024;15:1396124. doi: 10.3389/fimmu.2024.1396124
Fitzgerald NE, MacClain KL. Imaging characteristics of hemophagocytic lymphohistiocytosis. Pediatr Radiol. 2003;33:392–401. doi: 10.1007/s00247-003-0894-9
Schmidt MH, Sung L, Shuckett BM. Hemophagocytic Lymphohistiocytosis in Children: Abdominal US Findings within 1 Week of Presentation. Radiology. 2004;230:685–9. doi: 10.1148/radiol.2303030223
Malik P, Antonini L, Mannam P, et al. MRI Patterns in Pediatric CNS Hemophagocytic Lymphohistiocytosis. Am J Neuroradiol. 2021;42:2077–85. doi: 10.3174/ajnr.A7292
Shieh AC, Guler E, Smith DA, et al. Hemophagocytic Lymphohistiocytosis: A Primer for Radiologists. Am J Roentgenol. 2020;214:W11–9. doi: 10.2214/AJR.19.21788
Hur M, Kim YC, Lee KM, et al. Macrophage Activation Syndrome in a Child with Systemic Juvenile Rheumatoid Arthritis. J Korean Med Sci. 2005;20:695. doi: 10.3346/jkms.2005.20.4.695
Behrens EM, Beukelman T, Paessler M, et al. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34:1133–8.
Shekarchian F, Abadi MKA, Shariati MM. Clinical approach to a child with hemophagocytic lymphohistiocytosis and bilateral optic nerve head infiltration: A case report and brief literature review. Clin Case Rep. 2023;11:e7999. doi: 10.1002/ccr3.7999
Wang L, Suo L, Kou F, et al. Ocular Phenotypes in Patients With Hemophagocytic Lymphohistiocytosis: A Retrospective Analysis in a Single Center Over 7 Years. Am J Ophthalmol. 2023;253:119–31. doi: 10.1016/j.ajo.2023.05.011
García-Domínguez M. et al. Atypical eye manifestation in macrophage activation syndrome complicating systemic juvenile idiopathic arthritis //Alergia, Asma e Inmunología Pediátricas. – 2020. – Т. 29. – №. 2. – С. 66-70.
Higgins GC. Complications of Treatments for Pediatric Rheumatic Diseases. Pediatr Clin North Am. 2018;65:827–54. doi: 10.1016/j.pcl.2018.04.008
Gloor AD, Chollet L, Christ LA, et al. Takayasu arteritis: Prevalence and clinical presentation in Switzerland. PloS One. 2021;16:e0250025. doi: 10.1371/journal.pone.0250025
Saad R, Tsoi K, Onac IA, et al. Streptococcus-associated vasculitis: A role for antibiotic therapy? IDCases. 2021;24:e01071. doi: 10.1016/j.idcr.2021.e01071
Espinoza JL, Ai S, Matsumura I. New Insights on the Pathogenesis of Takayasu Arteritis: Revisiting the Microbial Theory. Pathog Basel Switz. 2018;7:E73. doi: 10.3390/pathogens7030073
Pedreira ALS, Santiago MB. Association between Takayasu arteritis and latent or active Mycobacterium tuberculosis infection: a systematic review. Clin Rheumatol. 2020;39:1019–26. doi: 10.1007/s10067-019-04818-5
Giancane G, Swart JF, Castagnola E, et al. Opportunistic infections in immunosuppressed patients with juvenile idiopathic arthritis: analysis by the Pharmachild Safety Adjudication Committee. Arthritis Res Ther. 2020;22:71. doi: 10.1186/s13075-020-02167-2
Vega-Briceño LE, Holmgren NL, Bertrand P, et al. Utility of Bronchoalveolar Lavage in Immunocompromised Children: Diagnostic Yield and Complications. Arch Bronconeumol Engl Ed. 2004;40:570–4. doi: 10.1016/S1579-2129(06)60377-7
Islabão AG, Trindade VC, da Mota LMH, et al. Managing Antiphospholipid Syndrome in Children and Adolescents: Current and Future Prospects. Pediatr Drugs. 2022;24:13–27. doi: 10.1007/s40272-021-00484-w
Janarthanan M, Antony T, Mohan R, et al. Disseminated tuberculosis with macrophage activation syndrome in a child with lupus nephritis. Sudan J Paediatr. 2021;190–4. doi: 10.24911/SJP.106-1614333951
Kim YW, Kwon BS, Lim SY, et al. Diagnostic value of bronchoalveolar lavage and bronchial washing in sputum-scarce or smear-negative cases with suspected pulmonary tuberculosis: a randomized study. Clin Microbiol Infect. 2020;26:911–6. doi: 10.1016/j.cmi.2019.11.013
Daelemans, Siel & Peeters, Linde & Wachter, Elke & Malfroot, Anne. (2019). Challenges in Diagnosing Mycobacterial Infections in Children.. 21. 194-199.
Wei M, Yongjie Zhao, Zhuoyu Qian, et al. Pneumonia caused by Mycobacterium tuberculosis. Microbes Infect. 2020;22:278–84. doi: 10.1016/j.micinf.2020.05.020
Ciftci E. Pulmonary involvement in childhood-onset systemic lupus erythematosus: a report of five cases. Rheumatology. 2004;43:587–91. doi: 10.1093/rheumatology/keh120
Sari MK, Satria CD, Arguni E. Predictors of Infection in Children with Systemic Lupus Erythematosus: A Single Center Study in Indonesia. Glob Pediatr Health. 2021;8:2333794X2110056. doi: 10.1177/2333794X211005609
Aygun D, Sahin S, Adrovic A, et al. The frequency of infections in patients with juvenile idiopathic arthritis on biologic agents: 1-year prospective study. Clin Rheumatol. 2019;38:1025–30. doi: 10.1007/s10067-018-4367-9
Restrepo-Gualteros SM, Gutierrez MJ, Villamil-Osorio M, et al. Challenges and Clinical Implications of the Diagnosis of Cytomegalovirus Lung Infection in Children. Curr Infect Dis Rep. 2019;21:24. doi: 10.1007/s11908-019-0681-x
Ramos JT, Romero CA, Belda S, et al. Clinical practice update of antifungal prophylaxis in immunocompromised children. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2019;32:410–25.
Matthews H, Rohde H, Wichmann D, et al. Invasive pulmonale Aspergillose. DMW - Dtsch Med Wochenschr. 2019;144:1218–22. doi: 10.1055/a-0817-7432
Tragiannidis A, Kyriakidis I, Zündorf I, et al. Invasive fungal infections in pediatric patients treated with tumor necrosis alpha (TNF-α) inhibitors. Mycoses. 2017;60:222–9. doi: 10.1111/myc.12576
Song H-M. Keeping up with the progress in the diagnosis and management of pediatric rheumatic diseases. World J Pediatr. 2020;16:1–4. doi: 10.1007/s12519-020-00340-w
Soni J, Baghel A, Chaudhary M, et al. Holter monitoring in pediatric patients with apparent life threatening events. J Pediatr Crit Care. 2019;6:15. doi: 10.21304/2019.0605.00526
Lazzerini PE, Capecchi PL, Guideri F, et al. Comparison of Frequency of Complex Ventricular Arrhythmias in Patients With Positive Versus Negative Anti-Ro/SSA and Connective Tissue Disease. Am J Cardiol. 2007;100:1029–34. doi: 10.1016/j.amjcard.2007.04.048
Munir S, Patil K, Miller E, et al. Juvenile idiopathic arthritis of the axial joints: a systematic review of the diagnostic accuracy and predictive value of conventional MRI. AJR Am J Roentgenol. 2014;202:199–210. doi: 10.2214/AJR.12.10475
Akhadov TA, Mitish VA, Bozhko OV, et al. Possibilities of magnetic resonance imaging in the diagnosis of acute aseptic sacroilitis in children. Diagn Radiol Radiother. 2022;13:72–80. doi: 10.22328/2079-5343-2022-13-2-72-80
Tutar E, Kutluk G, Bayrak NA, et al. What is the diagnostic utility of endoscopic scoring systems in children? Turk J Gastroenterol. 2009;24:22–9. doi: 10.4318/tjg.2013.0700
Frittoli RB, Vivaldo JF, Costallat LTL, et al. Gastrointestinal involvement in systemic lupus erythematosus: A systematic review. J Transl Autoimmun. 2021;4:100106. doi: 10.1016/j.jtauto.2021.100106
Kröner PT, Tolaymat OA, Bowman AW, et al. Gastrointestinal Manifestations of Rheumatological Diseases. Am J Gastroenterol. 2019;114:1441–54. doi: 10.14309/ajg.0000000000000260
Alharbi S. Gastrointestinal Manifestations in Patients with Systemic Lupus Erythematosus. Open Access Rheumatol Res Rev. 2022;Volume 14:243–53. doi: 10.2147/OARRR.S384256
Liu L, Liu L, Zhang L, et al. Case Report: A case of recurrent thrombosis in pediatric antiphospholipid syndrome associated with pediatric onset systemic lupus. Front Pediatr. 2023;10:1004053. doi: 10.3389/fped.2022.1004053
Galindo-Zavala R, Bou-Torrent R, Magallares-López B, et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr Rheumatol Online J. 2020;18:20. doi: 10.1186/s12969-020-0411-9
Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol Hoboken NJ. 2017;69:1521–37. doi: 10.1002/art.40137
Jaramillo D. What is the optimal imaging of osteonecrosis, Perthes, and bone infarcts? Pediatr Radiol. 2009;39 Suppl 2:S216-219. doi: 10.1007/s00247-009-1151-7
Gokcen N, Komac A, Tuncer F, et al. Risk factors of avascular necrosis in Takayasu arteritis: a cross sectional study. Rheumatol Int. 2022;42:529–34. doi: 10.1007/s00296-021-04909-0
Shine NP, Hamilton S, McShane DP. Takayasu’s arteritis and saddle nose deformity: a new association. J Laryngol Otol. 2006;120:59–62. doi: 10.1017/S0022215105004664
van Raalte DH, Diamant M. Steroid diabetes: from mechanism to treatment? Neth J Med. 2014;72:62–72.
Huber BM, Bolt IB, Sauvain M-J, et al. Adrenal insufficiency after glucocorticoid withdrawal in children with rheumatic diseases. Acta Paediatr Oslo Nor 1992. 2010;99:1889–93. doi: 10.1111/j.1651-2227.2010.01936.x
Ahmet A, Brienza V, Tran A, et al. Frequency and Duration of Adrenal Suppression Following Glucocorticoid Therapy in Children With Rheumatic Diseases. Arthritis Care Res. 2017;69:1224–30. doi: 10.1002/acr.23123
Conklin AI, Hong J. Obesity prevention in corticosteroid-treated patients: Use and effectiveness of strategies for weight management. Clin Obes. 2019;9:e12312. doi: 10.1111/cob.12312
Kalangos A, Christenson JT, Cikirikcioglu M, et al. Long-term outcome after surgical intervention and interventional procedures for the management of Takayasu’s arteritis in children. J Thorac Cardiovasc Surg. 2006;132:656–64. doi: 10.1016/j.jtcvs.2006.04.020
Gualtierotti R, Parisi M, Ingegnoli F. Perioperative Management of Patients with Inflammatory Rheumatic Diseases Undergoing Major Orthopaedic Surgery: A Practical Overview. Adv Ther. 2018;35:439–56. doi: 10.1007/s12325-018-0686-0
Fc A. The Portuguese Society of Rheumatology position paper on the use of biosimilars – 2017 update. 2017.
Atzeni F, Sebastiani M, Ricci C, et al. Position paper of Italian rheumatologists on the use of biosimilar drugs.
Abad Hernández MÁ, Andreu JL, Caracuel Ruiz MÁ, et al. Position Paper From the Spanish Society of Rheumatology on Biosimilar Drugs. Reumatol Clínica Engl Ed. 2015;11:269–78. doi: 10.1016/j.reumae.2015.03.012
Huizinga TWJ, Torii Y, Muniz R. Adalimumab Biosimilars in the Treatment of Rheumatoid Arthritis: A Systematic Review of the Evidence for Biosimilarity. Rheumatol Ther. 2021;8:41–61. doi: 10.1007/s40744-020-00259-8
Sahin S, Hopurcuoglu D, Bektas S, et al. Childhood-onset Takayasu arteritis: A 15-year experience from a tertiary referral center. Int J Rheum Dis. 2019;22:132–9. doi: 10.1111/1756-185X.13425
Aeschlimann FA, Eng SWM, Sheikh S, et al. Childhood Takayasu arteritis: disease course and response to therapy. Arthritis Res Ther. 2017;19:255. doi: 10.1186/s13075-017-1452-4
Sener S, Basaran O, Kaya Akca U, et al. Treatment of childhood-onset Takayasu arteritis: switching between anti-TNF and anti-IL-6 agents. Rheumatology. 2022;61:4885–91. doi: 10.1093/rheumatology/keac149
Sener S, Basaran O, Ozen S. Wind of Change in the Treatment of Childhood-Onset Takayasu Arteritis: a Systematic Review. Curr Rheumatol Rep. 2021;23:68. doi: 10.1007/s11926-021-01032-8
Department of Pediatric Rheumatology, Hacettepe University Faculty of Medicine,Ankara, Türkiye, Bayındır Y, Başaran Ö, et al. Vasculitis in Children. Turk Arch Pediatr. 2024;59:517–26. doi: 10.5152/TurkArchPediatr.2024.24181
Millan P, Gavcovich TB, Abitbol C. Childhood-onset Takayasu arteritis. Curr Opin Pediatr. 2022;34:223–8. doi: 10.1097/MOP.0000000000001113
Levy DM, Imundo LF. Nonsteroidal Anti-Inflammatory Drugs: A survey of practices and concerns of pediatric medical and surgical specialists and a summary of available safety data. Pediatr Rheumatol. 2010;8:7. doi: 10.1186/1546-0096-8-7
Litalien C., Jacqz-Aigrain E. Risks and benefits of nonsteroidal anti-inflammatory drugs in children //Paediatric drugs. – 2001. – Т. 3. – №. 11. – С. 817-858.
Hollingworth P. The use of non-steroidal anti-inflammatory drugs in paediatric rheumatic diseases //Rheumatology. – 1993. – Т. 32. – №. 1. – С. 73-77.
Guillaume-Czitrom S. Les anti-inflammatoires non stéroïdiens dans les rhumatismes inflammatoires chroniques de l’enfant [Non -steroidal anti-inflammatory drugs in chronic inflammatory arthritis in children]. La Lettre du Rhumatologue №379-380 - février-mars 2012.
Gupta P, Sachdev HP. Safety of oral use of nimesulide in children: systematic review of randomized controlled trials. Indian Pediatr. 2003 Jun;40(6):518-31. PMID: 12824661.
Todd PA, Sorkin EM. Diclofenac Sodium: A Reappraisal of its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs. 1988;35:244–85. doi: 10.2165/00003495-198835030-00004
Алексеева Е., Валиева С. Оценка эффективности, переносимости и безопасности нимесулида у детей с ювенильным артритом. Вопросы современной педиатрии. 2007;6(6):76-80.
Standing J. F. Diclofenac for acute pain in children: Pharmacokinetics and safety. – University of London, University College London (United Kingdom), 2007.
Saadoun D, Bura-Riviere A, Comarmond C, et al. French recommendations for the management of Takayasu’s arteritis. Orphanet J Rare Dis. 2021;16:311. doi: 10.1186/s13023-021-01922-1
Shetty AK, Stopa AR, Gedalia A. Low-dose methotrexate as a steroid-sparing agent in a child with Takayasu’s arteritis. Clin Exp Rheumatol. 1998;16:335–6.
Prey S, Paul C. Effect of folic or folinic acid supplementation on methotrexate-associated safety and efficacy in inflammatory disease: a systematic review. Br J Dermatol. 2009;160:622–8. doi: 10.1111/j.1365-2133.2008.08876.x
On behalf of the Rheumatology Italian Study Group, Ferrara G, Mastrangelo G, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol. 2018;16:46. doi: 10.1186/s12969-018-0255-8
Guti rrez-Su rez R., Burgos-Vargas R. The use of methotrexate in children with rheumatic diseases //Clinical and Experimental Rheumatology-Incl Supplements. – 2010. – Т. 28. – №. 5. – С. S122.
Hunt PG, Rose CD, McIlvain-Simpson G, et al. The effects of daily intake of folic acid on the efficacy of methotrexate therapy in children with juvenile rheumatoid arthritis. A controlled study. J Rheumatol 1997;24:2230–2.
Niehues T, Lankisch P. Recommendations for the Use of Methotrexate in Juvenile Idiopathic Arthritis: Pediatr Drugs. 2006;8:347–56. doi: 10.2165/00148581-200608060-00003
Pagnoux C., Goulet M. Role and place of methotrexate in vasculitis management //International Journal of Clinical Rheumatology. – 2009. – Т. 4. – №. 6. – С. 697.
Rheumatology and vasculitides. url: https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-04/PaedChp%2012%20Rheumatology_N_NEMLC-Oct%202022%20-%20Final.pdf.
Kang M, Lai J, Zhang D, et al. Clinical observations on infliximab treatment of infantile onset Takayasu arteritis. Pediatr Rheumatol. 2022;20:61. doi: 10.1186/s12969-022-00708-4
Mekinian A, Biard L, Dagna L, et al. Efficacy and safety of TNF-α antagonists and tocilizumab in Takayasu arteritis: multicentre retrospective study of 209 patients. Rheumatology. 2022;61:1376–84. doi: 10.1093/rheumatology/keab635
Kostik MM, Raupov RK, Suspitsin EN, et al. The Safety and Efficacy of Tofacitinib in 24 Cases of Pediatric Rheumatic Diseases: Single Centre Experience. Front Pediatr. 2022;10:820586. doi: 10.3389/fped.2022.820586
Mv P, Maikap D, Padhan P. Successful Use of Tofacitinib in Refractory Takayasu Arteritis: A Case Series. Mediterr J Rheumatol. 2023;34:356. doi: 10.31138/mjr.230929.su
Liang B, Luo X, Zhang Z, et al. Efficacy of upadacitinib in treating a paediatric case of refractory Takayasu arteritis. Rheumatology. 2025;keaf113. doi: 10.1093/rheumatology/keaf113
Алексеева Е.И., Крехова Е.А., Криулин И.А., Криулина Т.Ю., Дворяковская Т.М., Исаева К.Б., Чистякова Е.Г., Чомахидзе А.М., Ломакина О.Л., Фетисова А.Н., Чибисова К.В., Цулукия И.Т., Шингарова М.Ш., Ботова М.С., Кондратьева Н.М., Кокина М.Ю., Румянцев М.А. Опыт применения ингибитора янус-киназ упадацитиниба у детей с ревматическими болезнями. Вопросы практической педиатрии. 2024; 19(2): 59–79. DOI: 10.20953/1817-7646-2024-2-59-79.
Batu ED, Sönmez HE, Hazırolan T, et al. Tocilizumab treatment in childhood Takayasu arteritis: Case series of four patients and systematic review of the literature. Semin Arthritis Rheum. 2017;46:529–35. doi: 10.1016/j.semarthrit.2016.07.012
Bravo Mancheño B, Perin F, Guez Vázquez Del Rey MDMR, et al. Successful tocilizumab treatment in a child with refractory Takayasu arteritis. Pediatrics. 2012;130:e1720-1724. doi: 10.1542/peds.2012-1384
Nakaoka Y, Isobe M, Tanaka Y, et al. Long-term efficacy and safety of tocilizumab in refractory Takayasu arteritis: final results of the randomized controlled phase 3 TAKT study. Rheumatol Oxf Engl. 2020;59:2427–34. doi: 10.1093/rheumatology/kez630
Yamazaki K, Kikuchi M, Nozawa T, et al. PReS-FINAL-2189: Tocillizumab for patients with takayasu arteritis in childhood refractory to conventional therapy. Pediatr Rheumatol. 2013;11:O24, 1546-0096-11-S2-O24. doi: 10.1186/1546-0096-11-S2-O24
Mekinian A, Biard L, Dagna L, et al. Efficacy and safety of TNF-α antagonists and tocilizumab in Takayasu arteritis: Multicenter retrospective study of 209 patients. Rheumatol Oxf Engl. 2021;keab635. doi: 10.1093/rheumatology/keab635
Pazzola G, Muratore F, Pipitone N, et al. Rituximab therapy for Takayasu arteritis: a seven patients experience and a review of the literature. Rheumatol Oxf Engl. 2018;57:1151–5. doi: 10.1093/rheumatology/kex249
Hoyer BF, Mumtaz IM, Loddenkemper K, et al. Takayasu arteritis is characterised by disturbances of B cell homeostasis and responds to B cell depletion therapy with rituximab. Ann Rheum Dis. 2012;71:75–9. doi: 10.1136/ard.2011.153007
Ozen S, Duzova A, Bakkaloglu A, et al. Takayasu arteritis in children: preliminary experience with cyclophosphamide induction and corticosteroids followed by methotrexate. J Pediatr. 2007;150:72–6. doi: 10.1016/j.jpeds.2006.10.059
Sun Y, Ma L, Ma L, et al. Cyclophosphamide could be a better choice than methotrexate as induction treatment for patients with more severe Takayasu’s arteritis. Rheumatol Int. 2017;37:2019–26. doi: 10.1007/s00296-017-3847-6
Stern S, Clemente G, Reiff A, et al. Treatment of Pediatric Takayasu arteritis with infliximab and cyclophosphamide: experience from an American-Brazilian cohort study. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2014;20:183–8. doi: 10.1097/RHU.0000000000000106
Téllez Arévalo AM, Quaye A, Rojas-Rodríguez LC, et al. Synthetic Pharmacotherapy for Systemic Lupus Erythematosus: Potential Mechanisms of Action, Efficacy, and Safety. Medicina (Mex). 2022;59:56. doi: 10.3390/medicina59010056
Ogino MH, Tadi P. Cyclophosphamide. StatPearls. Treasure Island (FL): StatPearls Publishing 2024.
Carless P. A. et al. Proposal for the inclusion of mesna (sodium 2-mercaptoethane sulfonate) for the prevention of ifosfamide and cyclophosphamide (oxazaphosphorine cytotoxics) induced haemorrhagic cystitis //Geneva, Switzerland: 17th Expert Committee on the Selection and Use of Essential Medicines. – 2008.
Al Salloum AA. Cyclophosphamide therapy for lupus nephritis: poor renal survival in Arab children. Pediatr Nephrol. 2003;18:357–61. doi: 10.1007/s00467-003-1110-8
Medscape. mesna (Rx). https://reference.medscape.com/drug/mesnex-mesna-342126#0.
Teles KA, Medeiros-Souza P, Lima FAC, et al. Cyclophosphamide administration routine in autoimmune rheumatic diseases: a review. Rev Bras Reumatol Engl Ed. 2017;57:596–604. doi: 10.1016/j.rbre.2016.09.008
Sulieman SE, Metjian TA, Zaoutis TE, et al. Pneumocystis Pneumonia: Epidemiology and Options for Prophylaxis in Non-HIV Immunocompromised Pediatric Patients. Curr Fungal Infect Rep. 2014;8:45–55. doi: 10.1007/s12281-014-0177-y
Distler JHW. Primäres und sekundäres Raynaud-Phänomen. Z Für Rheumatol. 2008;67:211–9. doi: 10.1007/s00393-008-0282-9
Kobayashi M, Takano K, Kamizono J, et al. A serious case of primary Raynaud’s phenomenon in an infant. Clin Case Rep. 2018;6:2089–91. doi: 10.1002/ccr3.1819
https://pro.uptodatefree.ir/Show/13353.
Lamprecht P, Schnabel A, Gross WL. Efficacy of alprostadil and iloprost in digital necrosis due to secondary Raynaud’s phenomenon. Br J Rheumatol. 1998;37(6):703-704. [PubMed 9667634].
Aleksandrov AA, Kisliak OA, Leontyeva IV. Clinical guidelines on arterial hypertension diagnosis, treatment and prevention in children and adolescents. Syst Hypertens. 2020;17:7–35. doi: 10.26442/2075082X.2020.2.200126
Tullus K, Marks SD. Vasculitis in children and adolescents: clinical presentation, etiopathogenesis, and treatment. Paediatr Drugs. 2009;11:375–80. doi: 10.2165/11316120-000000000-00000
Koç R, Sönmez HE, Çakan M, et al. Drug reactions in children with rheumatic diseases receiving parenteral therapies: 9 years’ experience of a tertiary pediatric rheumatology center. Rheumatol Int. Published Online First: 21 December 2019. doi: 10.1007/s00296-019-04498-z
Watanabe Y, Yamaguchi Y. Drug allergy and autoimmune diseases. Allergol Int. 2022;71:179–84. doi: 10.1016/j.alit.2022.02.001
Felix MMR, Kuschnir FC, Boechat JL, et al. Recent findings on drug hypersensitivity in children. Front Allergy. 2024;5:1330517. doi: 10.3389/falgy.2024.1330517
Park JS, Suh DI. Drug Allergy in Children: What Should We Know? Clin Exp Pediatr. 2020;63:203–10. doi: 10.3345/kjp.2019.00675
Yazicioglu M. Approach to drug allergies in the childhood. Türk Pediatri Arş. 2014;49:99–103. doi: 10.5152/tpa.2014.1944
Paul F, Cartron G. Infusion-related reactions to rituximab: frequency, mechanisms and predictors. Expert Rev Clin Immunol. 2019;15:383–9. doi: 10.1080/1744666X.2019.1562905
Henter J-I. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100:2367–73. doi: 10.1182/blood-2002-01-0172
La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465–77. doi: 10.1182/blood.2018894618
Stephan J. L. et al. Reactive haemophagocytic syndrome in children with inflammatory disorders. A retrospective study of 24 patients //Rheumatology. – 2001. – Т. 40. – №. 11. – С. 1285-1292.
I.A.Kriulin IAK, Kriulin IA, National Medical Research Center for Children’s Health, Moscow, Russian Federation, et al. Hemophagocytic lymphohistiocytosis: mechanisms of development, clinical manifestations, and treatments. Vopr Prakt Pediatr. 2021;16:94–102. doi: 10.20953/1817-7646-2021-6-94-102
Ramanan A. V., Schneider R. Macrophage activation syndrome following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis //The Journal of rheumatology. – 2003. – Т. 30. – №. 2. – С. 401-403.
Bruck N. et al. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids //JCR: Journal of Clinical Rheumatology. – 2011. – Т. 17. – №. 1. – С. 23-27.
Zhang H, Yang S-W, Fu Y-C, et al. Cytokine storm and targeted therapy in hemophagocytic lymphohistiocytosis. Immunol Res. 2022;70:566–77. doi: 10.1007/s12026-022-09285-w
Криулин И.А., Алексеева Е.И., Шилькрот И.Ю., Дворяковская Т.М. Лечение вторичного гемофагоцитарного синдрома у пациентов с системным ювенильным идиопатическим артритом. Результаты когортного ретроспективного исследования. Вопросы практической педиатрии. 2022; 17(5): 7–19. DOI: 10.20953/1817-7646-2022-5-7-19.
Pelewicz K, Miśkiewicz P. Glucocorticoid Withdrawal—An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice. Diagnostics. 2021;11:728. doi: 10.3390/diagnostics11040728
Alves C, Robazzi TCV, Mendonça M. Withdrawal from glucocorticosteroid therapy: clinical practice recommendations. J Pediatr (Rio J). 2008;84:192–202. doi: 10.2223/JPED.1773
Кузьмина Н.И., Шох Б.П., Никишина И.П. Современный взгляд на системную глюкокортикостероидную терапию при ювенильном ревматоидном артрите //Научно-практическая ревматология. – 2000. – №. 2. – С. 56-62.
Mouy R. et al. Efficacy of cyclosporine A in the treatment of macrophage activation syndrome in juvenile arthritis: report of five cases //The Journal of pediatrics. – 1996. – Т. 129. – №. 5. – С. 750-754.
Georgiadou S, Gatselis NK, Stefos A, et al. Efficient management of secondary haemophagocytic lymphohistiocytosis with intravenous steroids and γ-immunoglobulin infusions. World J Clin Cases. 2019;7:3394–406. doi: 10.12998/wjcc.v7.i21.3394
Sen E. S., Clarke S. L. N., Ramanan A. V. Macrophage activation syndrome //The Indian Journal of Pediatrics. – 2016. – Т. 83. – №. 3. – С. 248-253.
Aydın F. et al. Comparison of baseline laboratory findings of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis and multisystem inflammatory syndrome in children //International Journal of Rheumatic Diseases. – 2021. – Т. 24. – №. 4. – С. 542-547.
Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259–68. doi: 10.1038/nrrheum.2015.179
Papa R, Natoli V, Caorsi R, et al. Successful treatment of refractory hyperferritinemic syndromes with canakinumab: a report of two cases. Pediatr Rheumatol. 2020;18:56. doi: 10.1186/s12969-020-00450-9
Bhat CS, Shetty R, Ramesh D, et al. Anakinra in Refractory Multisystem Inflammatory Syndrome in Children (MIS-C). Indian Pediatr. 2021;58:994–6. doi: 10.1007/s13312-021-2340-1
Phadke O, Rouster-Stevens K, Giannopoulos H, et al. Intravenous administration of anakinra in children with macrophage activation syndrome. Pediatr Rheumatol. 2021;19:98. doi: 10.1186/s12969-021-00585-3
Pal P, Bathia J, Giri PP, et al. Macrophage activation syndrome in pediatrics: 10 years data from an Indian center. Int J Rheum Dis. 2020;23:1412–6. doi: 10.1111/1756-185X.13915
Sönmez HE, Demir S, Bilginer Y, et al. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol. 2018;37:3329–35. doi: 10.1007/s10067-018-4095-1
Zhang Q, Zhao Y-Z, Ma H-H, et al. A study of ruxolitinib response–based stratified treatment for pediatric hemophagocytic lymphohistiocytosis. Blood. 2022;139:3493–504. doi: 10.1182/blood.2021014860
Sato S, Matsumoto H, Temmoku J, et al. A case of Takayasu arteritis complicated by refractory ulcerative colitis successfully treated with tofacitinib. Rheumatology. 2020;59:1773–5. doi: 10.1093/rheumatology/kez580
Rao S, Abzug MJ, Carosone-Link P, et al. Intravenous Acyclovir and Renal Dysfunction in Children: A Matched Case Control Study. J Pediatr. 2015;166:1462-1468.e4. doi: 10.1016/j.jpeds.2015.01.023
Kimberlin DW. Acyclovir Dosing in the Neonatal Period and Beyond. J Pediatr Infect Dis Soc. 2013;2:179–82. doi: 10.1093/jpids/pis138
Widasmara D, Firdausiya F. Disseminated Herpes Zoster on a Child with Systemic Lupus Erythematosus and Lupus Nephritis. Infect Drug Resist. 2021;Volume 14:2777–85. doi: 10.2147/IDR.S314220
Balfour HH, McMonigal KA, Bean B. Acyclovir therapy of varicella-zoster virus infections in immunocompromised patients. J Antimicrob Chemother. 1983;12:169–79. doi: 10.1093/jac/12.suppl_B.169
Whitley RJ. Herpes simplex virus in children. Curr Treat Options Neurol. 2002;4:231–7. doi: 10.1007/s11940-002-0040-2
Antiviral Drugs in Children and Adolescents. Pediatr Infect Dis. 2020;1:123–8. doi: 10.5005/jp-journals-10081-1221
Adler SP, Marshall B. Cytomegalovirus Infections. ;11.
Zhang S, Zhu Y, Jin Y, et al. Difference between Acyclovir and Ganciclovir in the Treatment of Children with Epstein–Barr Virus-Associated Infectious Mononucleosis. Evid Based Complement Alternat Med. 2021;2021:1–6. doi: 10.1155/2021/8996934
Chellapandian D, Das R, Zelley K, et al. Treatment of Epstein Barr virus-induced haemophagocytic lymphohistiocytosis with rituximab-containing chemo-immunotherapeutic regimens. Br J Haematol. 2013;162:376–82. doi: 10.1111/bjh.12386
Andrews A, Hickling P. Thrombosis associated with antiphospholipid antibody in juvenile chronic arthritis. Lupus. 1997;6:556–7. doi: 10.1177/096120339700600616
Thomas KN, Aggarwal A. Childhood rheumatic diseases: bites not only the joint, but also the heart. Clin Rheumatol. 2023;42:2703–15. doi: 10.1007/s10067-023-06621-9
Carcillo JA, Shakoory B, Castillo L. Secondary Hemophagocytic Lymphohistiocytosis, Macrophage Activation Syndrome, and Hyperferritinemic Sepsis-Induced Multiple-Organ Dysfunction Syndrome in the Pediatric ICU. In: Mastropietro CW, Valentine KM, eds. Pediatric Critical Care. Cham: Springer International Publishing 2019:245–55.
Лисицин А., Алексеева Е., Пинелис В., Баканов М., Валиева С., Бзарова Т. Опыт применения ибандроновой кислоты у больных с тяжелым течением ревматических болезней и системным остеопорозом. Вопросы современной педиатрии. 2010;9(1):116-121.
Yilmaz D., Ritchey A. K. Severe neutropenia in children: a single institutional experience //Journal of Pediatric Hematology/Oncology. – 2007. – Т. 29. – №. 8. – С. 513-518.
Segel, G. B., & Halterman, J. S. (2008). Neutropenia in pediatric practice. Pediatrics in review, 29(1), 12.
Jog NR, Young KA, Munroe ME, et al. Association of Epstein-Barr virus serological reactivation with transitioning to systemic lupus erythematosus in at-risk individuals. Ann Rheum Dis. 2019;78:1235–41. doi: 10.1136/annrheumdis-2019-215361
Demirkol D, Yildizdas D, Bayrakci B, et al. Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Crit Care. 2012;16:R52. doi: 10.1186/cc11256
El-Nawawy A, El-Kinany H, Hamdy El-Sayed M, et al. Intravenous Polyclonal Immunoglobulin Administration to Sepsis Syndrome Patients: A Prospective Study in a Pediatric Intensive Care Unit. J Trop Pediatr. 2005;51:271–8. doi: 10.1093/tropej/fmi011
Vassilopoulos A, McCormick W, Lakhani A. Update in Hyperferritinemic Syndromes: Recognition and Management - A Scoping Review. J Brown Hosp Med. 2022;1. doi: 10.56305/001c.37667
Henter J-I, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. doi: 10.1002/pbc.21039
Ladapo TA, Gajjar P, McCulloch M, et al. Impact of revascularization on hypertension in children with Takayasu’s arteritis-induced renal artery stenosis: a 21-year review. Pediatr Nephrol Berl Ger. 2015;30:1289–95. doi: 10.1007/s00467-015-3049-y
Reddy E, Robbs JV. Surgical management of Takayasu’s arteritis in children and adolescents. Cardiovasc J Afr. 2007;18:393–6.
Zhou Y, Feng Y, Zhang W, et al. Physical Exercise in Managing Takayasu Arteritis Patients Complicated With Cardiovascular Diseases. Front Cardiovasc Med. 2021;8:603354. doi: 10.3389/fcvm.2021.603354
Klepper SE. Exercise in pediatric rheumatic diseases. Curr Opin Rheumatol. 2008;20:619–24. doi: 10.1097/BOR.0b013e32830634ee
Alibaz-Oner F, Asmaz-Haliloglu Ö, Gogas-Yavuz D, et al. Vitamin D Levels in Takayasu’s Arteritis and a Review of the Literature on Vasculitides. J Clin Lab Anal. 2016;30:529–33. doi: 10.1002/jcla.21898
Yamamoto EA, Jørgensen TN. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front Immunol. 2020;10:3141. doi: 10.3389/fimmu.2019.03141
Ward LM. Glucocorticoid-Induced Osteoporosis: Why Kids Are Different. Front Endocrinol. 2020;11. doi: 10.3389/fendo.2020.00576
Alberta Health Services. Nutrition for Children Taking Steroids. URL: https://www.albertahealthservices.ca/assets/info/nutrition/if-nfs-nutrition-for-children-taking-steroids.pdf.
Emery HM, Bowyer SL, Sisung CE. Rehabilitation of the child with a rheumatic disease. Pediatr Clin North Am. 1995;42:1263–83. doi: 10.1016/s0031-3955(16)40062-3
Nicholls D, editor. Mobilizing knowledge in physiotherapy: critical reflections on foundations and practices. Abingdon, Oxon ; New York, NY: Routledge 2021.
Pountney TE, editor. Physiotherapy for children. Edinburgh ; New York: Butterworth-Heinemann/Elsevier 2007.
Oliveira DS, Shinjo SK, Silva MG, et al. Exercise in Takayasu Arteritis: Effects on Inflammatory and Angiogenic Factors and Disease-Related Symptoms. Arthritis Care Res. 2017;69:892–902. doi: 10.1002/acr.23011
Takken T, Van Brussel M, Engelbert RHH, et al. Exercise therapy in juvenile idiopathic arthritis. Cochrane Database Syst Rev. 2008;2010. doi: 10.1002/14651858.CD005954.pub2
Luttosch F, Baerwald C. Rehabilitation in der Rheumatologie. Internist. 2010;51:1239–45. doi: 10.1007/s00108-010-2626-1
Stucki G, Kroeling P. Physical therapy and rehabilitation in the management of rheumatic disorders. Best Pract Res Clin Rheumatol. 2000;14:751–71. doi: 10.1053/berh.2000.0111
Ansell BM, Chamberlain MA. 11 Children with chronic arthritis: the management of transition to adulthood. Baillières Clin Rheumatol. 1998;12:363–74. doi: 10.1016/S0950-3579(98)80023-X
Nascimento Leite M, Kamper SJ, O’Connell NE, et al. Physical activity and education about physical activity for chronic musculoskeletal pain in children and adolescents. Cochrane Database Syst Rev. 2023;2023. doi: 10.1002/14651858.CD013527.pub2
Kuntze G, Nesbitt C, Whittaker JL, et al. Exercise Therapy in Juvenile Idiopathic Arthritis: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2018;99:178-193.e1. doi: 10.1016/j.apmr.2017.05.030
Tarakci E, Arman N, Tarakci D, et al. Leap Motion Controller–based training for upper extremity rehabilitation in children and adolescents with physical disabilities: A randomized controlled trial. J Hand Ther. 2020;33:220-228.e1. doi: 10.1016/j.jht.2019.03.012
Heijstek MW, Ott de Bruin LM, Bijl M, et al. EULAR recommendations for vaccination in paediatric patients with rheumatic diseases. Ann Rheum Dis. 2011;70:1704–12. doi: 10.1136/ard.2011.150193
Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882
Jansen MHA, Rondaan C, Legger GE, et al. EULAR/PRES recommendations for vaccination of paediatric patients with autoimmune inflammatory rheumatic diseases: update 2021. Ann Rheum Dis. 2023;82:35–47. doi: 10.1136/annrheumdis-2022-222574
Кокина М.Ю., Фомина Д.С., Лебедкина М.C., Мутовина З.Ю., Жолобова Е.С., Курбанова С.Х., Наргизян А.К., Фетисова А.Н., Анджель А.Е., Дворяковская Т.М., Шилькрот И.Ю., Алексеева Е.И. Эффективность и безопасность применения двухкомпонентного препарата моноклональных антител к SARS-CoV-2 (тиксагевимаб + цилгавимаб) для доконтактной профилактики новой коронавирусной инфекции у иммунокомпрометированных пациентов детского возраста с ревматическими заболеваниями. Предварительные результаты первого в Российской Федерации проспективного наблюдательного когортного исследования. Вопросы практической педиатрии. 2023; 18(1): 16–26
Novelli, V., & Holzel, H. (1999). Safety and tolerability of fluconazole in children. Antimicrobial agents and chemotherapy, 43(8), 1955-1960.
Shovman O, Tamar S, Amital H, et al. Diverse patterns of anti-TNF-α-induced lupus: case series and review of the literature. Clin Rheumatol. 2018;37:563–8. doi: 10.1007/s10067-017-3884-2
Einarsson JT, Evert M, Geborek P, et al. Rituximab in clinical practice: dosage, drug adherence, Ig levels, infections, and drug antibodies. Clin Rheumatol. 2017;36:2743–50. doi: 10.1007/s10067-017-3848-6
Compagno N, Malipiero G, Cinetto F, et al. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 2014;5:626. doi: 10.3389/fimmu.2014.00626
Rodriguez MM, Wagner-Weiner L. Intravenous Immunoglobulin in Pediatric Rheumatology: When to Use It and What Is the Evidence. Pediatr Ann. 2017;46. doi: 10.3928/19382359-20161214-01
Scientific Centre of Children’s Health, Moscow, Russian Federation, Alexeeva EI, Denisova RV, et al. Intravenous Immunoglobulin in Pediatric Rheumatology Practice. Curr Pediatr Vopr Sovrem Pediatr. 2015;14:219–23. doi: 10.15690/vsp.v14i2.1290
Щербина А.Ю. Первичные иммунодефициты -реалии XXI века. Вопросы Гематологиионкологии И Иммунопатологии В Педиатрии 2016;15:8–9. doi:10.24287/1726-1708-2016-15-1-8-9.
Pan S, Yu H, Surti A, et al. Pharmacodynamics of rituximab on B lymphocytes in paediatric patients with autoimmune diseases. Br J Clin Pharmacol. 2019;85:1790–7. doi: 10.1111/bcp.13970
McAtee CL, Lubega J, Underbrink K, et al. Association of Rituximab Use With Adverse Events in Children, Adolescents, and Young Adults. JAMA Netw Open. 2021;4:e2036321. doi: 10.1001/jamanetworkopen.2020.36321
Smolewska E, Cebula B, Brózik H, et al. Relationship between impaired apoptosis of lymphocytes and distribution of dendritic cells in peripheral blood and synovial fluid of children with juvenile idiopathic arthritis. Arch Immunol Ther Exp (Warsz). 2008;56:283–9. doi: 10.1007/s00005-008-0030-5
Kaegi C, Wuest B, Schreiner J, et al. Systematic Review of Safety and Efficacy of Rituximab in Treating Immune-Mediated Disorders. Front Immunol. 2019;10:1990. doi: 10.3389/fimmu.2019.01990
Alexeeva EI, Valieva SI, Bzarova TM, et al. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin Rheumatol. 2011;30:1163–72. doi: 10.1007/s10067-011-1720-7
Khojah AM, Miller ML, Klein-Gitelman MS, et al. Rituximab-associated Hypogammaglobulinemia in pediatric patients with autoimmune diseases. Pediatr Rheumatol. 2019;17:61. doi: 10.1186/s12969-019-0365-y
Núñez Cuadros E, Calzada-Hernández J, Clemente D, et al. Correction to: Position statement of the Spanish society of pediatric rheumatology on infection screening, prophylaxis, and vaccination of pediatric patients with rheumatic diseases and immunosuppressive therapies: part 1 (screening). Eur J Pediatr. 2022;181:2355–2355. doi: 10.1007/s00431-022-04448-1
Papadopoulou C, Eleftheriou D. How do I ensure safe use of biological agents in children and adolescents with rheumatic diseases? Paediatr Child Health. 2014;24:264–8. doi: 10.1016/j.paed.2013.10.004
Prasad AN, Chaudhary S. Intravenous immunoglobulin in pediatrics: A review. Med J Armed Forces India. 2014;70:277–80. doi: 10.1016/j.mjafi.2013.05.011
Lucas GN. Clinical usage of intravenous immunoglobulin in children. Sri Lanka J Child Health. 2019;48:191. doi: 10.4038/sljch.v48i3.8751
Foran JM, Norton AJ, Micallef IN, et al. Loss of CD20 expression following treatment with rituximab (chimaeric monoclonal anti-CD20): a retrospective cohort analysis. Br J Haematol. 2001;114:881–3. doi: 10.1046/j.1365-2141.2001.03019.x
Zahran AM, Abdallah AM, Saad K, et al. Peripheral Blood B and T Cell Profiles in Children with Active Juvenile Idiopathic Arthritis. Arch Immunol Ther Exp (Warsz). 2019;67:427–32. doi: 10.1007/s00005-019-00560-7
Gurbanov A, Gün E, Botan E, et al. Intravenous Immunoglobulin Use in Pediatric Intensive Care: A Single-Center Experience. 2023.
Баранов А.А., Алексеева Е.И., Бзарова Т.М., et al. Протокол ведения пациентов с ювенильным артритом. Вопросы Современной Педиатрии. 2013;12:37–56.
Baddley JW, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [I]: anti-tumor necrosis factor-α agents). Clin Microbiol Infect. 2018;24:S10–20. doi: 10.1016/j.cmi.2017.12.025
Aizawa-Yashiro T, Oki E, Tsuruga K, et al. Intravenous immunoglobulin therapy leading to dramatic improvement in a patient with systemic juvenile idiopathic arthritis and severe pericarditis resistant to steroid pulse therapy. Rheumatol Int. 2012;32:1359–61. doi: 10.1007/s00296-010-1413-6
Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014;2:15. doi: 10.1186/2052-0492-2-15
De Vere-Tyndall A, Macauley D, Ansell BM. Disseminated intravascular coagulation complicating systemic juvenile chronic arthritis (”Still’s disease”). Clin Rheumatol. 1983;2:415. doi: 10.1007/BF02041564
McGrowder, D., Brown, P., Gordon, L., Budall, S., Irving, R., Alexander-Lindo, R., & Mhlanga, M. The Application of Thromboelastography in Clinical Practice.
Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46:10–67. doi: 10.1007/s00134-019-05878-6
Бзарова Т., Алексеева Е., Акулова С. Случай Эпштейна-Барр вирусной инфекции, протекавшей под маской системного варианта ювенильного ревматоидного артрита. Вопросы современной педиатрии. 2007;6(3):101-106.
Consolaro A, Giancane G, Alongi A, et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: an observational cohort study. Lancet Child Adolesc Health. 2019;3:255–63. doi: 10.1016/S2352-4642(19)30027-6
Shim GH. Treatment of congenital cytomegalovirus infection. Clin Exp Pediatr. 2023;66:384–94. doi: 10.3345/cep.2022.01032
Lin X, Wan Y, Liu Y. Efficacy of ganciclovir in the treatment of cytomegalovirus (CMV) infection in infants and its effect on inflammatory reaction and immune function. Am J Transl Res. 2023;15:6514–23.
Овсянкина Е.С, Губкина М.Ф., Панова Л.В., Юхименко Н.В. Методы скрининга туберкулезной инфекции у детей и их роль в формировании групп риска и диагностике заболевания. Российский педиатрический журнал. 2017; 20 (2): 108-115. DOI: http://dx.doi.org/10.18821/1560-9561-2017-20 (2): 108-115.
Wiger K., Høiby E. A., Wathne K. O. Infections in immunosuppressed children //Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, ny Raekke. – 2005. – Т. 125. – №. 9. – С. 1168-1172.
Acebo J. J. et al. Infections in Immunosuppressed Pediatric Patients //Pediatric Surgical Oncology. – Cham : Springer International Publishing, 2023. – С. 1-34.
Siberry GK, Abzug MJ, Nachman S, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children: Recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 2013;32:i. doi: 10.1097/01.inf.0000437856.09540.11
Allen UD. Management of infections in the immunocompromised child: General principles. LymphoSign J. 2016;3:87–98. doi: 10.14785/lymphosign-2016-0007
Patrick C. C. Clinical management of infections in immunocompromised infants and children //(No Title). – 2001.
Hurd A, Beukelman T. Infectious Complications in Juvenile Idiopathic Arthritis. Curr Rheumatol Rep. 2013;15:327. doi: 10.1007/s11926-013-0327-1
Sharma AP, Norozi K, Filler G, et al. Diagnosis of Pediatric Hypertension: European Society of Hypertension-recommended 24-hr vs. 24-hr-day-night Ambulatory Blood Pressure thresholds. ;23.
Бугун О. В., Рычкова Л. В., Долгих В. В. Двадцатичетырехчасовое мониторирование артериального давления в диагностике эссенциальной артериальной гипертензии в детском возрасте //Сибирский научный медицинский журнал. – 2003. – №. 2. – С. 49-53.
Nechytailo D. et al. Value of ambulatory blood pressure monitoring in the verification of arterial hypertension in school age children //georgian medical. – 2020. – С. 96.
Dolezalova P, Price-Kuehne FE, Özen S, et al. Disease activity assessment in childhood vasculitis: development and preliminary validation of the Paediatric Vasculitis Activity Score (PVAS). Ann Rheum Dis. 2013;72:1628–33. doi: 10.1136/annrheumdis-2012-202111
Бзарова Т.М., Щербаков П.Л., Алексеева Е.И., Чистякова Е.Г., and Валиева С.И.. “Лечение гастроэзофагеальной рефлюксной болезни у детей с юношеским артритом” Вопросы современной педиатрии, vol. 6, no. 4, 2007, pp. 17-22.
Pichler J, Ong C, Shah N, et al. Histopathological features of gastrointestinal mucosal biopsies in children with juvenile idiopathic arthritis. Pediatr Res. 2016;79:895–901. doi: 10.1038/pr.2016.27
Weber P, Brune T, Ganser G, et al. Gastrointestinal symptoms and permeability in patients with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2003;21:657–62.
Gryboski J. D. Peptic ulcer disease in children //Medical Clinics of North America. – 1991. – Т. 75. – №. 4. – С. 889-902.
Рубрикатор клинических рекомендаций. Язвенная болезнь желудка и/или двенадцатиперстной кишки. URL: https://cr.minzdrav.gov.ru/view-cr/388_3
Lobo L, Antunes D. Chest CT in infants and children. Eur J Radiol. 2013;82:1108–17. doi: 10.1016/j.ejrad.2011.12.006
Ramos JT, Romero CA, Belda S, et al. Clinical practice update of antifungal prophylaxis in immunocompromised children. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter 2019;32:410–25.
Park JW, Curtis JR, Kim MJ, et al. Pneumocystis pneumonia in patients with rheumatic diseases receiving prolonged, non-high-dose steroids—clinical implication of primary prophylaxis using trimethoprim–sulfamethoxazole. Arthritis Res Ther. 2019;21:207. doi: 10.1186/s13075-019-1996-6
García-Moreno J, Melendo-Pérez S, Martín-Gómez MT, et al. Pneumocystis jirovecii pneumonia in children. A retrospective study in a single center over three decades. Enfermedades Infecc Microbiol Clínica. 2020;38:111–8. doi: 10.1016/j.eimc.2019.05.005
Djawe K, Daly KR, Levin L, et al. Humoral Immune Responses to Pneumocystis jirovecii Antigens in HIV-Infected and Uninfected Young Children with Pneumocystis Pneumonia. PLoS ONE. 2013;8:e82783. doi: 10.1371/journal.pone.0082783
Müller F-M, Trusen A, Weig M. Clinical manifestations and diagnosis of invasive aspergillosis in immunocompromised children. Eur J Pediatr. 2002;161:563–74. doi: 10.1007/s00431-002-1041-6
Douglas AP, Smibert OliviaC, Bajel A, et al. Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021. Intern Med J. 2021;51:143–76. doi: 10.1111/imj.15591
Solovic I, Sester M, Gomez-Reino JJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–206. doi: 10.1183/09031936.00028510
Qu Y, Liu M, Sun X, et al. Development and evaluation of a triplex droplet digital PCR method for differentiation of M. tuberculosis, M. bovis and BCG. Front Microbiol. 2024;15:1397792. doi: 10.3389/fmicb.2024.1397792
Noguera-Julian A, Calzada-Hernández J, Brinkmann F, et al. Tuberculosis Disease in Children and Adolescents on Therapy With Antitumor Necrosis Factor-ɑ Agents: A Collaborative, Multicenter Paediatric Tuberculosis Network European Trials Group (ptbnet) Study. Clin Infect Dis. 2020;71:2561–9. doi: 10.1093/cid/ciz1138
Armbrust W, Kamphuis SSM, Wolfs TWF, et al. Tuberculosis in a nine-year-old girl treated with infliximab for systemic juvenile idiopathic arthritis. Rheumatology. 2004;43:527–9. doi: 10.1093/rheumatology/keh074
What’s New Adult and Adolescent Opportunistic Infection. AIDSinfo. https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-opportunistic-infection/392/whats-new (accessed 14 April 2020)
Pneumonia in Immunocompromised Patients: Overview, Causes of Pneumonia, HIV/AIDS. Published Online First: 23 March 2020.https://emedicine.medscape.com/article/807846-overview#a11.. (accessed 14 Apr 2020).
Siberry GK, Abzug MJ, Nachman S, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children: Recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 2013;32:i. doi: 10.1097/01.inf.0000437856.09540.11
Santos MJ, Canhão H, Conde M, et al. Portuguese recommendations for the use of biological therapies in children and adolescents with juvenile idiopathic arthritis--December 2011 update. Acta Reumatol Port. 2012;37:48–68.
Zhang Y, Milojevic D. Protecting Bone Health in Pediatric Rheumatic Diseases: Pharmacological Considerations. Pediatr Drugs. 2017;19:193–211. doi: 10.1007/s40272-017-0219-3
Денисова Р., Алексеева Е., Пинелис В., Баканов М., Валиева С., Бзарова Т., Исаева К., Морев С., Кузнецова Г. Эффективность и безопасность ибандроновой кислоты для внутривенного введения при тяжелом системном остеопорозе у больных ювенильным артритом. Вопросы современной педиатрии. 2011;10(6):83–88.
Kutilek S, Plasilova I, Langer J. Ibandronate in the treatment of pediatric osteoporosis. Bone Abstracts. Bioscientifica 2015.
Bachrach LK, Ward LM. Clinical Review: Bisphosphonate Use in Childhood Osteoporosis. J Clin Endocrinol Metab. 2009;94:400–9. doi: 10.1210/jc.2008-1531
Jcs Joint Working Group. Guideline for Management of Vasculitis Syndrome (JCS 2008) - Digest Version -: – Digest Version –. Circ J. 2011;75:474–503. doi: 10.1253/circj.CJ-88-0007
Petje G, Radler C, Aigner N, et al. Pharmacological management of aseptic osteonecrosis in children. Expert Opin Pharmacother. 2004;5:1455–62. doi: 10.1517/14656566.5.7.1455
Harari S, Paciocco G, Aramu S. Ear and nose involvement in systemic diseases. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2000;55:466–70.
Whittle, Vanessa & Gane, J & Cheetham, T. (2017). G169(P) Management of steroid induced diabetes in children: A national perspective. Archives of Disease in Childhood. 102. A69.1-A69. 10.1136/archdischild-2017-313087.168.
Hynes L, Saetes S, McGuire B, et al. Child and Family Adaptation to Juvenile Idiopathic Arthritis—A Systematic Review of the Role of Resilience Resources and Mechanisms. Front Psychol. 2019;10:2445. doi: 10.3389/fpsyg.2019.02445
Puxeddu I, Giori L, Rocchi V, et al. Hypersensitivity reactions during treatment with infliximab, etanercept, and adalimumab. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2012;108:123–4. doi: 10.1016/j.anai.2011.11.004
Алексеева Е., Валиева С., Бзарова Т., Семикина Е., Исаева К., Лисицин А., Денисова Р., Чистякова Е., Слепцова Т., Митенко Е. Эффективность и безопасность отечественного рекомбинантного человеческого гранулоцитарного колониестимулирующего фактора при нейтропениях, развивающихся на фоне анти-в клеточной и иммуносупрессивной терапии у больных ювенильным ревматоидным артритом. Вопросы современной педиатрии. 2010;9(4):94-100.
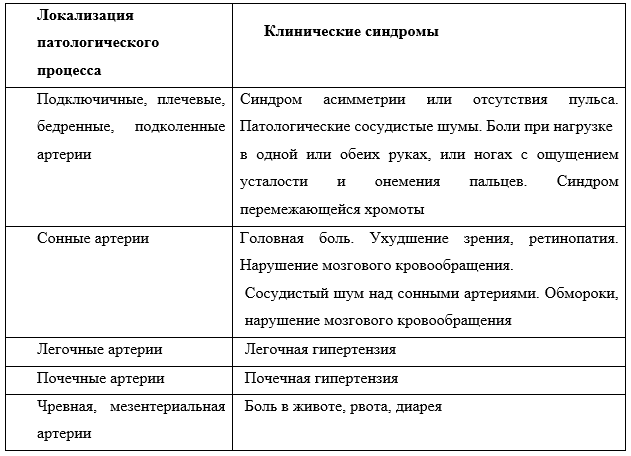
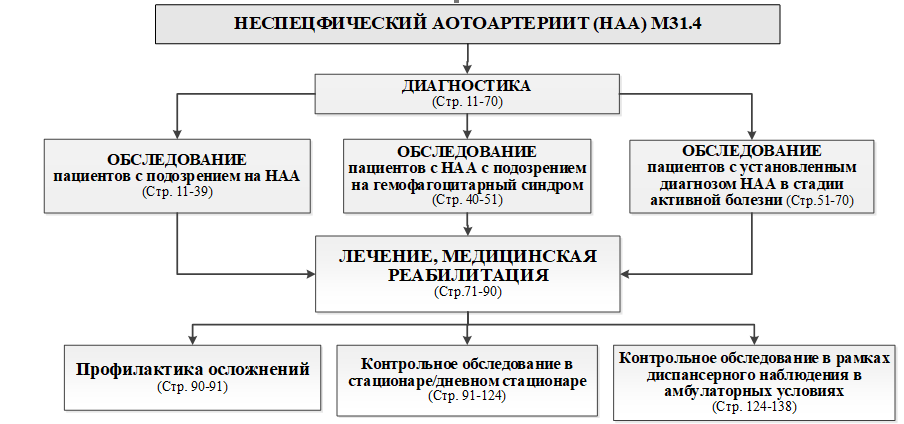 Схема 2
Схема 2