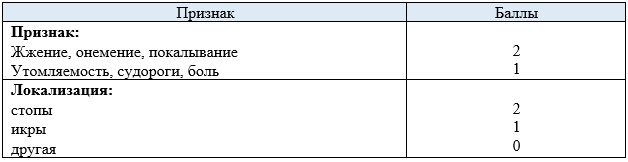
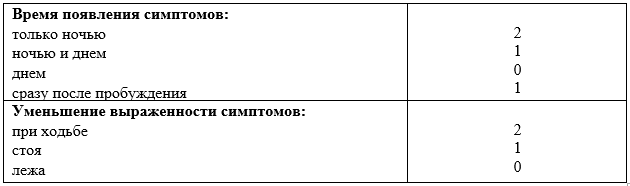
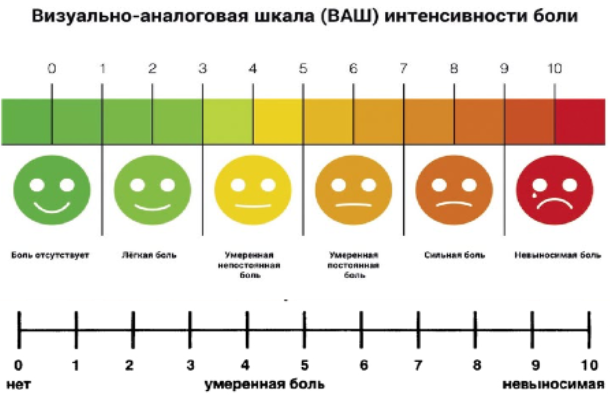
Гусев Е.И., Никифоров А.С. Неврологические симптомы, синдромы и болезни. Москва: ГЭОТАР-Медиа, 2006. Vol. 1184.
Рамазанов Г.Р. et al. Диагностика и лечение энцефалопатии Вернике: методические рекомендации. Москва: ГБУЗ «НИИ СП им. Н.В. Склифосовского ДЗМ», 2024. Vol. 35 с.
Медицинская токсикология. Национальное руководство / ed. Лужников Е.А. Москва: ГЭОТАР-Медиа, 2014. Vol. 952.
Sudulagunta S.R. et al. Posterior reversible encephalopathy syndrome(PRES). // Oxford Med. case reports. 2017. Vol. 2017, № 4. P. omx011.
Ando Y. et al. Posterior Reversible Encephalopathy Syndrome: A Review of the Literature // Intern. Med. 2022. Vol. 61, № 2. P. 7520–7521.
Ramazanov G.R. et al. Reversible Cerebral Vasoconstriction Syndrome // Russ. Sklifosovsky J. “Emergency Med. Care.” 2024. Vol. 13, № 3. P. 492–500.
Пирадов М.А., Супонева Н.А., Гришина Д.А. Полинейропатии: алгоритмы диагностики и лечения / ed. Попова Н.А. Москва: Горячая линия - Телеком, 2020. Vol. 248 с: ил.
Le Guennec L. et al. Toxic-metabolic encephalopathy in adults: Critical discussion and pragmatical diagnostic approach // Rev. Neurol. (Paris). 2022. Vol. 178, № 1–2. P. 93–104.
Pop-Busui R. et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association // Diabetes Care. 2017. Vol. 40, № 1. P. 136–154.
Dedov I.I. et al. Diabetes mellitus in Russian Federation: prevalence, morbidity, mortality, parameters of glycaemic control and structure of glucose lowering therapy according to the Federal Diabetes Register, status 2017 // Diabetes Mellit. 2018. Vol. 21, № 3. P. 144–159.
Маркизова Н.Ф. et al. Спирты. Санкт-Петербург: ООО «Издательство ФОЛИАНТ», 2004. Vol. 112.
Alexandrovsky V.N. et al. Acute Poisoning with Ethyl Alcohol (Alcoholic Coma) // Russ. Sklifosovsky J. “Emergency Med. Care.” 2019. Vol. 7, № 4. P. 357–365.
Sahu P., Verma H.K., Bhaskar L. Alcohol and alcoholism associated neurological disorders: Current updates in a global perspective and recent recommendations. // World J. Exp. Med. 2025. Vol. 15, № 1. P. 100402.
Shormanova N.S., Kulikov S. V. MORPHOLOGICAL CHARACTERISTICS OF MAIN BRAIN STRUCTURES IN HEALTH AND UNDER CONDITIONS OF CHRONIC ALCOHOLIC INTOXICATION // Univ. proceedings. Volga Reg. Med. Sci. 2017. № 3.
Gaikova O.N. et al. Morphological Changes in the Brain During Toxic Injury // J. Biomed. 2024. Vol. 20, № 3. P. 28–31.
Hammoud N., Jimenez-Shahed J. Chronic Neurologic Effects of Alcohol // Clin. Liver Dis. 2019. Vol. 23, № 1. P. 141–155.
Kawarabuki K. et al. Marchiafava–Bignami disease: magnetic resonance imaging findings in corpus callosum and subcortical white matter // Eur. J. Radiol. 2003. Vol. 48, № 2. P. 175–177.
King J.D., Rosner M.H. Osmotic Demyelination Syndrome // Am. J. Med. Sci. 2010. Vol. 339, № 6. P. 561–567.
ALEXANDROV Y.I. et al. Effect of ethanol on hippocampal neurons depends on their behavioural specialization // Acta Physiol. Scand. 1993. Vol. 149, № 1. P. 105–115.
BUTTERWORTH R.F. Pathophysiologic mechanisms responsible for the reversible (thiamine‐responsive) and irreversible (thiamine non‐responsive) neurological symptoms of Wernicke’s encephalopathy // Drug Alcohol Rev. 1993. Vol. 12, № 3. P. 315–322.
Thomson A.D. THE ROYAL COLLEGE OF PHYSICIANS REPORT ON ALCOHOL: GUIDELINES FOR MANAGING WERNICKE’S ENCEPHALOPATHY IN THE ACCIDENT AND EMERGENCY DEPARTMENT // Alcohol Alcohol. 2002. Vol. 37, № 6. P. 513–521.
Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, D.C.: National Academies Press, 1998.
Постников С.С. et al. Нейротоксичность лекарств // Качественная клиническая практика. 2017. № 4. P. 68–72.
Ситкали И.В., Чиричкин А.С., Колоколов О.В. Поражение периферической нервной системы, ассоциированное с приемом лекарственных средств // Лечащий врач. 2019. Vol. 5. P. 19–25.
Litvincev B.S. et al. Neurological aspects of modern drug addiction // Toxicol. Rev. 2020. № 2. P. 25–29.
Cunha-Oliveira T., Rego A.C., Oliveira C.R. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. // Brain Res. Rev. 2008. Vol. 58, № 1. P. 192–208.
Blasel S. et al. Toxic Leukoencephalopathy after Heroin Abuse without Heroin Vapor Inhalation // Clin. Neuroradiol. 2010. Vol. 20, № 1. P. 48–53.
Rando J. et al. Methadone overdose causing acute cerebellitis and multi-organ damage // Am. J. Emerg. Med. 2016. Vol. 34, № 2. P. 343.e1-343.e3.
Haghighi-Morad M. et al. Methadone-induced encephalopathy: a case series and literature review // BMC Med. Imaging. 2020. Vol. 20, № 1. P. 6.
Nylander E. et al. The effects of morphine, methadone, and fentanyl on mitochondria: A live cell imaging study // Brain Res. Bull. 2021. Vol. 171. P. 126–134.
Morgan J.P. Amphetamine and Methamphetamine During the 1990s // Pediatr. Rev. 1992. Vol. 13, № 9. P. 330–333.
Seiden LS, Kleven MS. Methamphetamine and related drugs: toxicity and resulting behavioral changes in response to pharmacological probes // NIDA Reseach Monogr. 1989. Vol. 94. P. 146–160.
Logothetis J. Desoxyn Therapy for Nocturnal Seizures // Neurology. 1955. Vol. 5, № 4. P. 236–236.
Хоффман Р. et al. Экстренная медицинская помощь при отравлениях. Москва: Практика, 2010. Vol. 1140.
Sinenchenko A.G. et al. Epidemiology of acute poisonings with gammahydroxybutyric acid in Saint Petersburg (according to data of a multidisciplinary hospital) // Toxicol. Rev. 2021. № 2. P. 33–40.
Loban I.E., Gorbacheva T. V., Bychkov V.A. The chemical toxicological investigation of gamma-hydroxybutyric acid in biological objects and the interpretation of the results of the analysis // Sud. Ekspert. 2018. Vol. 61, № 5. P. 25.
Sinenchenko A.G. et al. Neurophysiological characteristics of patients with severe acute poisoning with 1,4-butanediol, complicated with delirium // Anesteziol. i Reanimatol. 2021. № 6. P. 68.
Илларионова Е.А., Сыроватский И.П. Химико-токсикологический анализ тяжелых металлов: учебное пособие. Иркутск: ИГМУ, 2016. Vol. 58.
Andrade V.M., Aschner M., Marreilha Dos Santos A.P. Neurotoxicity of Metal Mixtures. // Adv. Neurobiol. 2017. Vol. 18. P. 227–265.
Ijomone O.M. et al. Epigenetic influence of environmentally neurotoxic metals. // Neurotoxicology. 2020. Vol. 81. P. 51–65.
Li X.-L. et al. Toxic encephalopathy induced by radix Sophorae tonkinensis // Acta Neurol. Belg. 2022. Vol. 122, № 3. P. 855–858.
Wen Z. et al. Case Report: Toxic encephalopathy caused by repeated inhalation of liquid sealant // Front. Public Heal. 2022. Vol. 10.
Santos-Sacramento L. et al. Human neurotoxicity of mercury in the Amazon: A scoping review with insights and critical considerations // Ecotoxicol. Environ. Saf. 2021. Vol. 208. P. 111686.
Alan H.B. Wu. Tietz Clinical Guide to Laboratory Tests. 4th ed. . St. Louis, Mo: Saunders/Elsevier, 2006. Vol. 1857.
Broussard L. Disposition of Toxic Drugs and Chemicals in Man, Seventh Edition. Randall C. Baselt. Foster City, CA: Biomedical Publications, 2004, 1250 pp., $139.50, hardcover. ISBN 09626523-6-9. // Clin. Chem. 2005. Vol. 51, № 3. P. 680–680.
Lin C.-C. et al. Acute encephalopathy following arsenic trioxide for metastatic urothelial carcinoma // Urol. Oncol. Semin. Orig. Investig. 2008. Vol. 26, № 6. P. 659–661.
Vahidnia A., van der Voet G.B., de Wolff F.A. Arsenic neurotoxicity — A review // Hum. Exp. Toxicol. 2007. Vol. 26, № 10. P. 823–832.
Dolbec K., Dobbs M.R., Ibraheem M. Toxin-Induced Cerebellar Disorders. // Neurol. Clin. 2020. Vol. 38, № 4. P. 843–852.
Goldstein G.W. Evidence that lead acts as a calcium substitute in second messenger metabolism // Neurotoxicology. 1993. Vol. 14, № 2–3. P. 97–102.
Markovac J., Goldstein G.W. Lead activates protein kinase C in immature rat brain microvessels // Toxicol. Appl. Pharmacol. 1988. Vol. 96, № 1. P. 14–23.
Simons T.J.B. CELLULAR INTERACTIONS BETWEEN LEAD AND CALCIUM // Br. Med. Bull. 1986. Vol. 42, № 4. P. 431–434.
Brookes P.S. et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle // Am. J. Physiol. Physiol. 2004. Vol. 287, № 4. P. C817–C833.
Кошкина В.С. et al. Клинико-токсикологическая характеристика свинца и его соединений // Медицинские новости. 2013. Vol. 1, № 220. P. 20–25.
Chuang H.-Y., Chao K.-Y., Tsai S.-Y. Reversible neurobehavioral performance with reductions in blood lead levels–A prospective study on lead workers // Neurotoxicol. Teratol. 2005. Vol. 27, № 3. P. 497–504.
de Souza A. et al. Adult lead encephalopathy // Neurol. Res. 2013. Vol. 35, № 1. P. 54–58.
Sainio M.A. Neurotoxicity of solvents. 2015. P. 93–110.
Bukowska B., Mokra K., Michałowicz J. Benzo[a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. // Int. J. Mol. Sci. 2022. Vol. 23, № 11.
van Valen E. et al. Chronic solvent-induced encephalopathy: European consensus of neuropsychological characteristics, assessment, and guidelines for diagnostics // Neurotoxicology. 2012. Vol. 33, № 4. P. 710–726.
Wang Y., Du P. Acute organic solvent toxic encephalopathy: A case report and literature review // Biomed. Reports. 2024. Vol. 21, № 5. P. 163.
Rajveer Kaur, Gurjot Kaur Mavi, Shweta Raghav. Pesticides Classification and Its Impact on Environment // Int.J.Curr.Microbiol.App.Sci. 2019. Vol. 8, № 03. P. 1889–1897.
Racchiusa S. et al. Posterior reversible encephalopathy syndrome (PRES) and infection: a systematic review of the literature // Neurol. Sci. 2019. Vol. 40, № 5. P. 915–922.
Singhal A.B. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome as Syndromes of Cerebrovascular Dysregulation // Contin. Lifelong Learn. Neurol. 2021. Vol. 27, № 5. P. 1301–1320.
Frontera J.A. Metabolic Encephalopathies in the Critical Care Unit // Contin. Lifelong Learn. Neurol. 2012. Vol. 18. P. 611–639.
Avola G., Pezzini A. Treatment-Related Reversible Cerebral Vasoconstriction Syndrome // J. Clin. Med. 2024. Vol. 13, № 19. P. 5930.
Ducros A. et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients // Brain. 2007. Vol. 130, № 12. P. 3091–3101.
Favrelière S. et al. Drugs associated with reversible cerebral vasoconstriction syndrome: A pharmacovigilance study in vigiBase ® // Cephalalgia. 2024. Vol. 44, № 8.
Constant dit Beaufils P. et al. Drug-induced reversible cerebral vasoconstriction syndrome: Lessons from the real world // Cephalalgia. 2025. Vol. 45, № 1.
Favreliere S. et al. A reply, drug-induced reversible cerebral vasoconstriction syndrome: Lessons from the real world // Cephalalgia. 2025. Vol. 45, № 1.
Жулев Н.М. et al. Невропатии: Руководство для врачей / ed. Н.М. Жулев. Санкт-Петербург: СПбМАПО, 2005. Vol. 416.
Левин О.С. Полиневропатии: Клиническое руководство. Москва: МИА, 2016. Vol. 480.
Петрова Н.Н. Алкогольная полинейропатия в терапевтической практике // Терапия. 2017. Vol. 5. P. 85–94.
Jones M.R. et al. Drug-Induced Peripheral Neuropathy: A Narrative Review // Curr. Clin. Pharmacol. 2020. Vol. 15, № 1. P. 38–48.
Cashman C.R., Höke A. Mechanisms of distal axonal degeneration in peripheral neuropathies // Neurosci. Lett. 2015. Vol. 596. P. 33–50.
Литвинцев Б.С. et al. Поражение периферической нервной системы у лиц молодого возраста при наркомании // Вестник Российской Военно-медицинской академии. 2014. Vol. 4, № 48. P. 24–28.
Андреева Г.О. et al. Спинальная неврология: учебное пособие / ed. Одинак М.М. Санкт-Петербург: СпецЛит, 2018. Vol. 430.
Федеральный научный центр гигиены им. Ф.Ф. Эрисмана. Гигиеническая классификация пестицидов по степени опасности: методические рекомендации. Москва, 2001. Vol. 19.
Карасева Е.И., Бутвиловский В.Э. Ядовитые грибы и растения: учебно-методическое пособие. Минск: БГМУ, 2014. Vol. 88.
Pigarova E.A., Dzeranova L.K. Metabolic mechanisms of development and compensation of osmotic stress in the brain // Obe. Metab. 2017. Vol. 14, № 4. P. 73–76.
Nardone R., Brigo F., Trinka E. Acute Symptomatic Seizures Caused by Electrolyte Disturbances // J. Clin. Neurol. 2016. Vol. 12, № 1. P. 21.
Sell J., Ramirez S., Partin M. Parathyroid Disorders. // Am. Fam. Physician. 2022. Vol. 105, № 3. P. 289–298.
Hansen B.-A., Bruserud Ø. Hypomagnesemia in critically ill patients // J. Intensive Care. 2018. Vol. 6, № 1. P. 21.
Дедов И.И., Шестакова М.В. Осложнения сахарного диабета: лечение и профилактика. Москва: МИА, 2017. Vol. 743.
Dedov I.I. et al. Diabetes mellitus type 1 in adults // Diabetes Mellit. 2020. Vol. 23, № 1S. P. 42–114.
Redondo M.J. Genetics of Type 1A Diabetes // Recent Prog. Horm. Res. 2001. Vol. 56, № 1. P. 69–90.
Atkinson M.A. The Pathogenesis and Natural History of Type 1 Diabetes // Cold Spring Harb. Perspect. Med. 2012. Vol. 2, № 11. P. a007641–a007641.
Antvorskov J.C. et al. Dietary gluten and the development of type 1 diabetes // Diabetologia. 2014. Vol. 57, № 9. P. 1770–1780.
Yukina M.Y. et al. The hypoglycemic syndrome (insulinoma): pathogenesis, etiology, laboratory diagnosis (review, part 1) // Probl. Endocrinol. 2017. Vol. 63, № 4. P. 245–256.
Mohseni S. Neurologic damage in hypoglycemia. 2014. P. 513–532.
Sultana D.R., Shahin A.D., Md. Jawadul H. Measurement of oxidative stress and total antioxidant capacity in hyperthyroid patients following treatment with carbimazole and antioxidant // Heliyon. 2022. Vol. 8, № 1. P. e08651.
Sahin L. et al. Hyperthyroidism leads learning and memory impairment possibly via GRIN2B expression alterations // Brain Res. 2023. Vol. 1802. P. 148209.
Madhusudhan U. et al. Brain-Derived Neurotrophic Factor-Mediated Cognitive Impairment in Hypothyroidism // Cureus. 2022.
Chen Y. et al. Detrimental effects of hypercortisolism on brain structure and related risk factors // Sci. Rep. 2020. Vol. 10, № 1. P. 12708.
Knezevic E. et al. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders // Cells. 2023. Vol. 12, № 23. P. 2726.
Bal B.S. et al. Nutritional deficiencies after bariatric surgery // Nat. Rev. Endocrinol. 2012. Vol. 8, № 9. P. 544–556.
Handzlik‐Orlik G. et al. Nutrition Management of the Post–Bariatric Surgery Patient // Nutr. Clin. Pract. 2015. Vol. 30, № 3. P. 383–392.
Gollobin C., Marcus W.Y. Bariatric Beriberi // Obes. Surg. 2002. Vol. 12, № 3. P. 309–311.
Stroh C., Meyer F., Manger T. Beriberi, a Severe Complication after Metabolic Surgery - Review of the Literature // Obes. Facts. 2014. Vol. 7, № 4. P. 246–252.
Lakhani S. V. et al. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients // Nutr. Res. 2008. Vol. 28, № 5. P. 293–298.
Chamorro A.J. et al. Differences Between Alcoholic and Nonalcoholic Patients With Wernicke Encephalopathy: A Multicenter Observational Study // Mayo Clin. Proc. 2017. Vol. 92, № 6. P. 899–907.
Vileikyte L. et al. Diabetic Peripheral Neuropathy and Depressive Symptoms // Diabetes Care. 2005. Vol. 28, № 10. P. 2378–2383.
DiAntonio A. Axon degeneration: mechanistic insights lead to therapeutic opportunities for the prevention and treatment of peripheral neuropathy // Pain. 2019. Vol. 160, № 1. P. S17–S22.
Mizukami H., Osonoi S. Pathogenesis and Molecular Treatment Strategies of Diabetic Neuropathy Collateral Glucose-Utilizing Pathways in Diabetic Polyneuropathy. // Int. J. Mol. Sci. 2020. Vol. 22, № 1.
Smith S. et al. Pathogenesis of Distal Symmetrical Polyneuropathy in Diabetes // Life. 2022. Vol. 12, № 7. P. 1074.
Kim B., Feldman E.L. Insulin resistance in the nervous system // Trends Endocrinol. Metab. 2012. Vol. 23, № 3. P. 133–141.
Feldman E.L. et al. Diabetic neuropathy // Nat. Rev. Dis. Prim. 2019. Vol. 5, № 1. P. 41.
Malik R.A. et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy // Diabetologia. 2005. Vol. 48, № 3. P. 578–585.
Tomlinson D.R., Gardiner N.J. Glucose neurotoxicity // Nat. Rev. Neurosci. 2008. Vol. 9, № 1. P. 36–45.
Potter C.G. et al. Hypoglycemic neuropathy in experimental diabetes // J. Neurol. Sci. 1988. Vol. 88, № 1–3. P. 293–301.
Heckmann J.G. et al. Hypoglycemic sensorimotor polyneuropathy associated with insulinoma // Muscle Nerve. 2000. Vol. 23, № 12. P. 1891–1894.
Спирин Н.Н., Никанорова Т.Ю. Полинейропатия у пациентов с заболеваниями щитовидной железы. 2016. Vol. 21, № 4. P. 26–30.
Gupta N. et al. Peripheral and central nervous system involvement in recently diagnosed cases of hypothyroidism: An electrophysiological study // Ann. Med. Health Sci. Res. 2016. Vol. 6, № 5. P. 261.
Oblaukhova V.I., Svetlana D.N., Mustafina S. V. Polyneuropathy on the background of thyrotoxicosis with thiamazole drug treatment // Clin. Exp. Thyroidol. 2018. Vol. 14, № 3. P. 156–161.
Garrido M.J.M. et al. Cushing’s paraneoplastic syndrome as first manifestation of an adenocarcinoma of unknown origin // Clin. Transl. Oncol. 2006. Vol. 8, № 8. P. 621–623.
Eskandari D. et al. A Case Report of Cushing’s Disease Presenting With Psychosis and Muscle Weakness Postpartum // J. Investig. Med. High Impact Case Reports. 2023. Vol. 11.
Bouattour N. et al. Vitamin B12 deficiency neuropathy: A clinical and electrophysiological study // Neurophysiol. Clin. 2018. Vol. 48, № 3. P. 130.
Nisar S. et al. Thiamine deficiency‐related neuropathy: A reversible entity from an endemic area // Eur. J. Neurol. 2024. Vol. 31, № 3.
Muhamad R. et al. The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review // Nutrients. 2023. Vol. 15, № 13. P. 2823.
Fei S. et al. Vitamin D deficiency increases the risk of diabetic peripheral neuropathy in elderly type 2 diabetes mellitus patients by predominantly increasing large-fiber lesions // Diabetes Res. Clin. Pract. 2024. Vol. 209. P. 111585.
Богомолова Е.В. et al. Социально-демографический анализ пациентов с алкогольной энцефалопатией и дисциркуляторной энцефалопатией. 2012. Vol. 8, № 2. P. 413–416.
Дамулин И.В. Алкогольная дегенерация мозжечка // Российский медицинский журнал. 2005. Vol. 2. P. 44–47.
Damulin I., Strutsenko A., Bychenko V. CENTRAL PONTINE MYELINOLYSIS // Vrach. 2018. Vol. 29, № 1.
Дамулин И.В., Струценко А.А. Болезнь (синдром) Маркиафавы-Биньями // Российский медицинский журнал. 2016. Vol. 22, № 6. P. 332–336.
Савченко Е.А. et al. МР-семиотика синдрома Вернике-Корсакова у пациентов с ВИЧ // Международный неврологический журнал. 2016. Vol. 3, № 81. P. 76–80.
Claudio Lopes Chaves L. et al. A Cluster of Polyneuropathy and Wernicke-Korsakoff Syndrome in a Bariatric Unit // Obes. Surg. 2002. Vol. 12, № 3. P. 328–334.
COOK C.C.H., HALLWOOD P.M., THOMSON A.D. B VITAMIN DEFICIENCY AND NEUROPSYCHIATRIC SYNDROMES IN ALCOHOL MISUSE // Alcohol Alcohol. 1998. Vol. 33, № 4. P. 317–336.
Lapergue B. et al. Diffusion weighted imaging of cerebellar lesions in Wernicke’s encephalopathy // J. Neuroradiol. 2006. Vol. 33, № 2. P. 126–128.
Harper C.G., Giles M., Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. // J. Neurol. Neurosurg. Psychiatry. 1986. Vol. 49, № 4. P. 341–345.
Kim W.J., Kim M.M. Wernicke’s Encephalopathy Presenting with Bilateral Complete Horizontal and Downward Gaze Palsy in a Malnourished Patient // Korean J. Ophthalmol. 2017. Vol. 31, № 4. P. 372.
Arts N., Walvoort S., Kessels R. Korsakoff’s syndrome: a critical review // Neuropsychiatr. Dis. Treat. 2017. Vol. Volume 13. P. 2875–2890.
Spencer M.R., Garnett M.F., Miniño A.M. Drug Overdose Deaths in the United States, 2002–2022. NCHS Data Brief, no 491. Hyattsville, 2024.
Ангельчева О.И., Зиновьева О.Е., Яхно Н.Н. Нервно-мышечные нарушения при хроническом алкоголизме: Учебное пособие. Москва: МЕДпресс-информ, 2009. Vol. 176.
Green S., Holton A. Drug-induced peripheral neuropathy // Adverse Drug React. Bull. 2016. Vol. 300, № 1. P. 1159–1162.
Литвинцев Б.С. Поражение нервной системы при наркомании. Санкт-Петербург: ВМедА, 2018. Vol. 160.
Бабанов С.А., Бараева Р.А. Профессиональные полинейропатии: дифференциальный диагноз, особенности фармакотерапии // Врач. 2014. Vol. 4. P. 13–19.
Гребеньков С.В. et al. Профессиональная полиневропатия: современный взгляд на проблему в России и за рубежом. Обзор литературы // Гигиена и санитария. 2019. Vol. 98, № 6. P. 631–635.
Шевченко О.И., Катаманова Е.В., Лахман О.Л. Особенности психопатологических изменений у больных с хронической ртутной интоксикацией // Доктор.Ру. 2015. Vol. 8–9, № 109–110. P. 59–64.
Константинова Т.Н. et al. Клинические случаи профессиональной хронической марганцевой интоксикации // Медицина труда и промышленная экология. 2009. Vol. 1. P. 27–31.
Akram K. et al. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey // J. Diabetes Complications. 2006. Vol. 20, № 6. P. 402–408.
Pivonello R. et al. Neuropsychiatric disorders in Cushing’s syndrome // Front. Neurosci. 2015. Vol. 9.
Антонова К.В. et al. Гипотиреоз и неврологические нарушения // Эффективная фармакотерапия. 2023. Vol. 19, № 3. P. 42–50.
Tapper E.B. et al. A risk score to predict the development of hepatic encephalopathy in a population‐based cohort of patients with cirrhosis // Hepatology. 2018. Vol. 68, № 4. P. 1498–1507.
Orman E.S. et al. Trends in Characteristics, Mortality, and Other Outcomes of Patients With Newly Diagnosed Cirrhosis. // JAMA Netw. open. 2019. Vol. 2, № 6. P. e196412.
Potnis A., VanMeter S., Stange J. Prevalence of Hepatic Encephalopathy from a Commercial Medical Claims Database in the United States // Int. J. Hepatol. 2021. Vol. 2021. P. 1–6.
Wang J.-Y. et al. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China. // World J. Gastroenterol. 2013. Vol. 19, № 30. P. 4984–4991.
Häussinger D. et al. Hepatic encephalopathy // Nat. Rev. Dis. Prim. 2022. Vol. 8, № 1. P. 43.
Murray A.M. et al. Cognitive impairment in hemodialysis patients is common // Neurology. 2006. Vol. 67, № 2. P. 216–223.
Alleman C.J.M. et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature // Diabetes Res. Clin. Pract. 2015. Vol. 109, № 2. P. 215–225.
Ziegler D. et al. Epidemiology of polyneuropathy in diabetes and prediabetes. 2014. P. 3–22.
Dimitropoulos G. Cardiac autonomic neuropathy in patients with diabetes mellitus // World J Diabetes. 2014. Vol. 5, № 1. P. 17.
Beghi E. et al. Hypothyroidism and polyneuropathy. // J. Neurol. Neurosurg. Psychiatry. 1989. Vol. 52, № 12. P. 1420–1423.
Cakir M. et al. Musculoskeletal manifestations in patients with thyroid disease // Clin. Endocrinol. (Oxf). 2003. Vol. 59, № 2. P. 162–167.
Duyff R.F. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study // J. Neurol. Neurosurg. Psychiatry. 2000. Vol. 68, № 6. P. 750–755.
Vasconcellos L.F.R. et al. Hoffman’s syndrome: pseudohypertrophic myopathy as initial manifestation of hypothyroidism. Case report // Arq. Neuropsiquiatr. 2003. Vol. 61, № 3B. P. 851–854.
Madariaga M.G. et al. Polymyositis-Like Syndrome in Hypothyroidism: Review of Cases Reported Over the Past Twenty-Five Years // Thyroid. 2002. Vol. 12, № 4. P. 331–336.
Сахаров А.В. Наркология: Учебное пособие. Чита: РИЦ ЧГМА, 2018. Vol. 252.
Гофман А.Г. Клиническая наркология. – 2-е изд. . Москва: ООО “Издательство МИА,” 2017. Vol. 376.
Инструментальная диагностика в неврологии: руководство для врачей / ed. под ред. Литвиненко И.В. О.М.М. Санкт-Петербург: СпецЛит, 2022. Vol. 334.
Chandrakumar A., Bhardwaj A., ‘t Jong G.W. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis // J. Basic Clin. Physiol. Pharmacol. 2019. Vol. 30, № 2. P. 153–162.
Oudman E. et al. Wernicke-Korsakoff syndrome despite no alcohol abuse: A summary of systematic reports // J. Neurol. Sci. 2021. Vol. 426. P. 117482.
Ota Y. et al. Comprehensive review of Wernicke encephalopathy: pathophysiology, clinical symptoms and imaging findings // Jpn. J. Radiol. 2020. Vol. 38, № 9. P. 809–820.
Thomson A.D. et al. Review * Wernicke’s encephalopathy revisited * Translation of the case history section of the original manuscript by Carl Wernicke “Lehrbuch der Gehirnkrankheiten fur Aerzte and Studirende” (1881) with a commentary // Alcohol Alcohol. 2008. Vol. 43, № 2. P. 174–179.
Fei G. -q. et al. Clinical Characteristics and MR Imaging Features of Nonalcoholic Wernicke Encephalopathy // Am. J. Neuroradiol. 2008. Vol. 29, № 1. P. 164–169.
Zuccoli G., Pipitone N. Neuroimaging Findings in Acute Wernicke’s Encephalopathy: Review of the Literature // Am. J. Roentgenol. 2009. Vol. 192, № 2. P. 501–508.
Manzo G. et al. MR Imaging Findings in Alcoholic and Nonalcoholic Acute Wernicke’s Encephalopathy: A Review // Biomed Res. Int. 2014. Vol. 2014. P. 1–12.
Zuccoli G. et al. Wernicke Encephalopathy: MR Findings at Clinical Presentation in Twenty-Six Alcoholic and Nonalcoholic Patients // Am. J. Neuroradiol. 2007. Vol. 28, № 7. P. 1328–1331.
Sechi G., Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management // Lancet Neurol. 2007. Vol. 6, № 5. P. 442–455.
Сиволап Ю.П., Дамулин И.В. Энцефалопатия Вернике и корсаковский психоз: клинико-патогенетические соотношения, диагностика и лечение // Журнал неврологии и психиатрии им. С.С. Корсакова. Спецвыпуски. 2013. Vol. 6, № 2. P. 20–26.
Курсов С.В. Кокаиновая интоксикация // Медицина неотложных состояний . 2020. Vol. 16, № 5. P. 33–44.
Mead J., Parrott A. Mephedrone and MDMA: A comparative review // Brain Res. 2020. Vol. 1735. P. 146740.
Breijyeh Z. et al. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses // Toxins (Basel). 2021. Vol. 13, № 2. P. 117.
Hofer K.E. et al. Acute toxicity associated with the recreational use of the novel dissociative psychoactive substance methoxphenidine // Clin. Toxicol. 2014. Vol. 52, № 10. P. 1288–1291.
Собенников В.С., Черняк Н.Б. Методические рекомендации для студентов к клиническому практическому занятию по теме: «Психические расстройства и расстройства поведения, вызванные употреблением психоактивных веществ». Иркутстк: ИГМУ, 2021. Vol. 45.
Addiction Medicine: Federal Guidelines / ed. by N.N. Ivanets, M.A. Vinnikova. 3rd edition, revised and enlarged // Addiction Medicine: Federal Guidelines / ed. by N.N. Ivanets, M.A. Vinnikova. 3rd edition, revised and enlarged / ed. Ivanets N.N., Vinnikova M.A. OOO «GEOTAR-Media» Publishing Group, 2024. P. 1–848.
Ana Paula Perestrelo et al. Chronic Copper Sulfate Poisoning // Eur. J. Case Reports Intern. Med. 2021. № LATEST ONLINE.
Chen P. et al. Iron and manganese-related CNS toxicity: mechanisms, diagnosis and treatment // Expert Rev. Neurother. 2019. Vol. 19, № 3. P. 243–260.
Ruczaj A., Brzóska M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system: A critical review of current data // J. Appl. Toxicol. 2023. Vol. 43, № 1. P. 66–88.
Iqubal A. et al. Environmental neurotoxic pollutants: review // Environ. Sci. Pollut. Res. 2020. Vol. 27, № 33. P. 41175–41198.
Lanphear B.P. et al. Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis // Environ. Health Perspect. 2005. Vol. 113, № 7. P. 894–899.
George J. et al. Informal gold miners with mercury toxicity: Novel asymmetrical neurological presentations // South African Med. J. 2023. Vol. 113, № 12. P. 20.
Harada M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution // Crit. Rev. Toxicol. 1995. Vol. 25, № 1. P. 1–24.
Ratnaike R.N. Acute and chronic arsenic toxicity // Postgrad. Med. J. 2003. Vol. 79, № 933. P. 391–396.
Lucchini R.G., Hashim D. Tremor secondary to neurotoxic exposure. 2015. P. 241–249.
Müller D., Desel H. Common Causes of Poisoning // Dtsch. Arztebl. Int. 2013.
Sriram K. et al. Biological effects of inhaled crude oil vapor V. Altered biogenic amine neurotransmitters and neural protein expression // Toxicol. Appl. Pharmacol. 2022. Vol. 449. P. 116137.
McKnight S., Hack N. Toxin-Induced Parkinsonism // Neurol. Clin. 2020. Vol. 38, № 4. P. 853–865.
Общероссийская общественная организация «Ассоциация врачей-офтальмологов», Общероссийская общественная организация «Общество офтальмологов России». Клинические рекомендации: Атрофия и аномалии зрительного нерва. 2022.
Офтальмология: национальное руководство. 2ое изд. / ed. ред. С.Э. Аветисова Е.А.Е.Л.К.М. [и др. . Москва: ГЭОТАР-Медиа, 2018. Vol. 904.
Roach E. Diagnosis and Management of Neurocutaneous Syndromes // Semin. Neurol. 1988. Vol. 8, № 01. P. 83–96.
Ostroumova O.D. et al. Drug-induced toxic optic neuropathy // Vestn. oftal’mologii. 2020. Vol. 136, № 4. P. 156.
Mohney B.G., Young R.C., Diehl N. Incidence and Associated Endocrine and Neurologic Abnormalities of Optic Nerve Hypoplasia // JAMA Ophthalmol. 2013. Vol. 131, № 7. P. 898.
Belskaya G.N., Sakharova E. V. Alcoholic polyneuropathy. Clinical forms and pathogenetically based approaches to therapy // Meditsinskiy Sov. = Med. Counc. 2021. № 10. P. 94–99.
Julian T. et al. Alcohol-related peripheral neuropathy: a systematic review and meta-analysis // J. Neurol. 2019. Vol. 266, № 12. P. 2907–2919.
Зиновьева О.Е., Ангельчева О.И. Вопросы патогенеза и лечения алкогольной полиневропатии // Неврология, нейропсихиатрия, психосоматика. 2004. Vol. 9, № 1. P. 45–50.
Строков И.А. et al. Острая алкогольная полиневропатия // Неврологический журнал. 2004. Vol. 9, № 1. P. 45–50.
Chubykina S.V., Tatarinova M.Y., Avakyan G.G. Mechanisms of platinum-induced peripheral neuropathy in cancer patients // Zhurnal Nevrol. i psikhiatrii im. S.S. Korsakova. 2023. Vol. 123, № 7. P. 19.
Литвинцев Б.С. Поражение нервной системы при наркомании: особенности симптоматики и неврологических осложнений // Вестник Российской Военно-медицинской академии. 2015. Vol. 1, № 49. P. 95–100.
Koszewicz M. et al. The impact of chronic co-exposure to different heavy metals on small fibers of peripheral nerves. A study of metal industry workers // J. Occup. Med. Toxicol. 2021. Vol. 16, № 1. P. 12.
Zavaliy L.B. et al. Diagnosis and treatment of persons with acute thallium poisoning // Toxicol. Reports. 2021. Vol. 8. P. 277–281.
Al Bshabshe A., Alfaifi M., Alsayed A.F. Black widow spider bites experience from tertiary care center in Saudi Arabia. // Avicenna J. Med. 2017. Vol. 7, № 2. P. 51–53.
Rusmili M.R.A. et al. Variations in neurotoxicity and proteome profile of Malayan krait (Bungarus candidus) venoms // PLoS One. 2019. Vol. 14, № 12. P. e0227122.
Bickler P.E. et al. Neuromuscular Weakness and Paralysis Produced by Snakebite Envenoming: Mechanisms and Proposed Standards for Clinical Assessment // Toxins (Basel). 2023. Vol. 15, № 1. P. 49.
Sanaei-Zadeh H. Spider Bite in Iran // Electron. Physician. 2017. Vol. 9, № 7. P. 4703–4707.
Godoy D.A. et al. Neurological and Systemic Manifestations of Severe Scorpion Envenomation // Cureus. 2021.
O. Collaço R. de C. et al. Scorpion venom increases acetylcholine release by prolonging the duration of somatic nerve action potentials // Neuropharmacology. 2019. Vol. 153. P. 41–52.
Chiang F., Castillo M. Seastrokes: A New Threat for North Carolina Swimmers? // Neuroradiol. J. 2014. Vol. 27, № 4. P. 499–502.
Katikou P. et al. An Updated Review of Tetrodotoxin and Its Peculiarities // Mar. Drugs. 2022. Vol. 20, № 1. P. 47.
Lonati D. et al. Foodborne Botulism: Clinical Diagnosis and Medical Treatment // Toxins (Basel). 2020. Vol. 12, № 8. P. 509.
Karakasi M. V et al. Conium maculatum intoxication: Literature review and case report on hemlock poisoning. // Forensic Sci. Rev. 2019. Vol. 31, № 1. P. 23–36.
Alizadeh A. et al. Black henbane and its toxicity - a descriptive review. // Avicenna J. phytomedicine. 2014. Vol. 4, № 5. P. 297–311.
Trabulus S., Altiparmak M.R. Clinical features and outcome of patients with amatoxin-containing mushroom poisoning // Clin. Toxicol. 2011. Vol. 49, № 4. P. 303–310.
Li H. et al. Mushroom Poisoning Outbreaks — China, 2022 // China CDC Wkly. 2023. Vol. 5, № 3. P. 45–50.
Gummin D.D. et al. 2019 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report // Clin. Toxicol. 2020. Vol. 58, № 12. P. 1360–1541.
Wennig R. et al. Mushroom Poisoning // Dtsch. Arztebl. Int. 2020.
Govorushko S. et al. Poisoning associated with the use of mushrooms: A review of the global pattern and main characteristics // Food Chem. Toxicol. 2019. Vol. 128. P. 267–279.
Yu X. Six groups of poisonous mushrooms: classified according to clinical symptoms // Highlights Sci. Eng. Technol. 2022. Vol. 19. P. 216–222.
Diaz J.H. Syndromic diagnosis and management of confirmed mushroom poisonings // Crit. Care Med. 2005. Vol. 33, № 2. P. 427–436.
Ott A. et al. Diabetes mellitus and the risk of dementia // Neurology. 1999. Vol. 53, № 9. P. 1937–1937.
Peila R., Rodriguez B.L., Launer L.J. Type 2 Diabetes, APOE Gene, and the Risk for Dementia and Related Pathologies // Diabetes. 2002. Vol. 51, № 4. P. 1256–1262.
Palta P. et al. Magnitude of Cognitive Dysfunction in Adults with Type 2 Diabetes: A Meta-analysis of Six Cognitive Domains and the Most Frequently Reported Neuropsychological Tests Within Domains // J. Int. Neuropsychol. Soc. 2014. Vol. 20, № 3. P. 278–291.
van den Berg E. et al. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition // Biochim. Biophys. Acta - Mol. Basis Dis. 2009. Vol. 1792, № 5. P. 470–481.
Yaffe K. et al. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. // J. Nutr. Health Aging. 2006. Vol. 10, № 4. P. 293–295.
Jurado-Flores M., Warda F., Mooradian A. Pathophysiology and Clinical Features of Neuropsychiatric Manifestations of Thyroid Disease // J. Endocr. Soc. 2022. Vol. 6, № 2.
Старостина Е.Г. Диабетическая нейропатия: некоторые вопросы дифференциальной диагностики и системной терапии болевого синдрома // РМЖ. 2017. Vol. 22. P. 1665–1676.
Ziegler D. et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: International expert consensus recommendations // Diabetes Res. Clin. Pract. 2022. Vol. 186. P. 109063.
Pop-Busui R. et al. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy // ADA Clin. Compend. 2022. Vol. 2022, № 1. P. 1–32.
Xu F. et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c // Diabetol. Metab. Syndr. 2014. Vol. 6, № 1. P. 139.
Ткачева О.Н., Верткин А.Л. Диабетическая автономная нейропатия. Руководство для врачей. Москва: ГЭОТАР-Медиа, 2009. Vol. 176.
Куцало А.Л. et al. Особенности бинокулярной динамической пупиллометрии у больных сахарным диабетом II типа // Практическая медицина. 2018. Vol. 16, № 5. P. 162–167.
Galvin R. et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy // Eur. J. Neurol. 2010. Vol. 17, № 12. P. 1408–1418.
Wijdicks E.F.M. Why you may need a neurologist to see a comatose patient in the ICU // Crit. Care. 2016. Vol. 20, № 1. P. 193.
Yatsko K. et al. Glasgow coma scale and full outline of unresponsiveness. Multicenter validation study in Russia // J. Neurol. Sci. 2023. Vol. 455. P. 122679.
Piradov M.A. et al. Full Outline of UnResponsiveness (FOUR) scale: translation and linguistic and cultural adaptation of the Russian language version // Ann. Clin. Exp. Neurol. 2019. Vol. 13, № 4.
Piradov M.A. et al. Full Outline of UnResponsiveness (FOUR) Scale: a Multicenter Validation Study of the Psychometric Properties of the Approved Russian Version // Gen. Reanimatol. 2024. Vol. 20, № 3. P. 15–21.
Ramazanov G.R. et al. Wernicke encephalopathy // Russ. Neurol. J. 2024. Vol. 29, № 2. P. 34–42.
Катаманова Е.В. et al. Нарушения высших психических функций при энцефалопатии различного генеза // Acta Biomed. Sci. 2012. Vol. 1. P. 26–31.
Котельникова А.В. et al. Психометрическая апробация скрининговых методик диагностики когнитивного статуса на выборке пациентов, перенесших ишемический инсульт // Вестник восстановительной медицины. 2023. Vol. 22, № 2. P. 32–41.
Gupta A. et al. Validity of Montreal Cognitive Assessment to Detect Cognitive Impairment in Individuals with Type 2 Diabetes // Diabetes Ther. 2024. Vol. 15, № 5. P. 1155–1168.
11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes—2019 // Diabetes Care. 2019. Vol. 42, № Supplement_1. P. S124–S138.
Bril V., Perkins B.A. Validation of the Toronto Clinical Scoring System for Diabetic Polyneuropathy // Diabetes Care. 2002. Vol. 25, № 11. P. 2048–2052.
Bastyr E.J., Price K.L., Bril V. Development and validity testing of the neuropathy total symptom score-6: Questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy // Clin. Ther. 2005. Vol. 27, № 8. P. 1278–1294.
Xiong Q. et al. The Diagnostic Value of Neuropathy Symptom and Change Score, Neuropathy Impairment Score and Michigan Neuropathy Screening Instrument for Diabetic Peripheral Neuropathy // Eur. Neurol. 2015. Vol. 74, № 5–6. P. 323–327.
Ismail-Beigi F. et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial // Lancet. 2010. Vol. 376, № 9739. P. 419–430.
Pop-Busui R. et al. Impact of Glycemic Control Strategies on the Progression of Diabetic Peripheral Neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort // Diabetes Care. 2013. Vol. 36, № 10. P. 3208–3215.
Jaiswal M. et al. Peripheral Neuropathy in Adolescents and Young Adults With Type 1 and Type 2 Diabetes From the SEARCH for Diabetes in Youth Follow-up Cohort // Diabetes Care. 2013. Vol. 36, № 12. P. 3903–3908.
Spallone V. et al. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy // Diabet. Med. 2012. Vol. 29, № 5. P. 578–585.
Young M.J. et al. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population // Diabetologia. 1993. Vol. 36, № 2. P. 150–154.
Petrova M.M. et al. Диагностика нейропатической боли: шкалы и вопросники // Sib. Med. Rev. 2020. № 3. P. 61–69.
Ramazanov G.R. et al. Clinical Cases of Wernicke Encephalopathy // Russ. Sklifosovsky J. “Emergency Med. Care.” 2020. Vol. 9, № 2. P. 292–297.
Frank L.L. Thiamin in Clinical Practice // J. Parenter. Enter. Nutr. 2015. Vol. 39, № 5. P. 503–520.
Whitfield K.C. et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs // Ann. N. Y. Acad. Sci. 2018. Vol. 1430, № 1. P. 3–43.
Donnino M.W. et al. Coronary artery bypass graft surgery depletes plasma thiamine levels // Nutrition. 2010. Vol. 26, № 1. P. 133–136.
Manzanares W., Hardy G. Thiamine supplementation in the critically ill // Curr. Opin. Clin. Nutr. Metab. Care. 2011. Vol. 14, № 6. P. 610–617.
Donnino M.W. et al. Thiamine deficiency in critically ill patients with sepsis // J. Crit. Care. 2010. Vol. 25, № 4. P. 576–581.
254. Frank T. et al. Assessment of Thiamin Status in Chronic Renal Failure Patients, Transplant Recipients and Hemodialysis Patients Receiving a Multivitamin Supplementation // Int. J. Vitam. Nutr. Res. 2000. Vol. 70, № 4. P. 159–166.
Dancy M. et al. Blood thiamine and thiamine phosphate ester concentrations in alcoholic and non-alcoholic liver diseases. // BMJ. 1984. Vol. 289, № 6437. P. 79–82.
Singarayar E.K., Josephrajan T.A., Jayabalan P. Relationship between blood glucose levels and diabetic neuropathy examination score in patients with diabetes mellitus // Int. J. Res. Med. Sci. 2025. Vol. 13, № 4. P. 1577–1581.
Zhang Y. et al. Estimated glucose disposal rate predicts the risk of diabetic peripheral neuropathy in type 2 diabetes: A 5‐year follow‐up study // J. Diabetes. 2024. Vol. 16, № 5.
Amelia R. et al. Early Detection of Diabetic Peripheral Neuropathy in Diabetic Patients: A Cross-Sectional Study // Curr. Diabetes Rev. 2025. Vol. 21, № 2.
Fleseriu M. et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update // Lancet Diabetes Endocrinol. 2021. Vol. 9, № 12. P. 847–875.
de Oliveira A.M. et al. Imaging Patterns of Toxic and Metabolic Brain Disorders // RadioGraphics. 2019. Vol. 39, № 6. P. 1672–1695.
Berisavac I. et al. How to recognize and treat metabolic encephalopathy in Neurology intensive care unit // Neurol. India. 2017. Vol. 65, № 1. P. 123.
Antunez E. et al. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. // Am. J. Roentgenol. 1998. Vol. 171, № 4. P. 1131–1137.
Jasne A.S. et al. Cerebellar Hippocampal and Basal Nuclei Transient Edema with Restricted diffusion (CHANTER) Syndrome // Neurocrit. Care. 2019. Vol. 31, № 2. P. 288–296.
Mallikarjun K.S. et al. Neuroimaging Findings in CHANTER Syndrome: A Case Series // Am. J. Neuroradiol. 2022. Vol. 43, № 8. P. 1136–1141.
McKinney A.M. et al. Acute Hepatic Encephalopathy: Diffusion-Weighted and Fluid-Attenuated Inversion Recovery Findings, and Correlation with Plasma Ammonia Level and Clinical Outcome // Am. J. Neuroradiol. 2010. Vol. 31, № 8. P. 1471–1479.
U-King-Im J.M. et al. Acute Hyperammonemic Encephalopathy in Adults: Imaging Findings // Am. J. Neuroradiol. 2011. Vol. 32, № 2. P. 413–418.
Hegde A.N. et al. Differential Diagnosis for Bilateral Abnormalities of the Basal Ganglia and Thalamus // RadioGraphics. 2011. Vol. 31, № 1. P. 5–30.
Benson J.C. et al. Delineation of microhemorrhage in acute hepatic encephalopathy using susceptibility-weighted imaging // Eur. J. Radiol. 2016. Vol. 85, № 3. P. 629–634.
Kang E.G. et al. Diffusion MR Imaging of Hypoglycemic Encephalopathy // Am. J. Neuroradiol. 2010. Vol. 31, № 3. P. 559–564.
Fujioka M. et al. Specific Changes in Human Brain After Hypoglycemic Injury // Stroke. 1997. Vol. 28, № 3. P. 584–587.
Yedavalli V. et al. Beyond the embolus: “do not miss” diffusion abnormalities of ischaemic and non-ischaemic neurological disease // Insights Imaging. 2017. Vol. 8, № 6. P. 573–580.
Degnan A.J., Levy L.M. Neuroimaging of Rapidly Progressive Dementias, Part 2: Prion, Inflammatory, Neoplastic, and Other Etiologies // Am. J. Neuroradiol. 2014. Vol. 35, № 3. P. 424–431.
Hattingen E. et al. Wernicke encephalopathy // Neurology. 2016. Vol. 87, № 18. P. 1956–1957.
Loh Y. Restricted Diffusion of the Splenium in Acute Wernicke’s Encephalopathy // J. Neuroimaging. 2005. Vol. 15, № 4. P. 373–375.
Sabatini J.S. et al. Wernicke’s encephalopathy with chorea: Neuroimaging findings // Dement. Neuropsychol. 2016. Vol. 10, № 4. P. 370–372.
Blanco M. et al. CT and MR imaging findings in methanol intoxication. // AJNR. Am. J. Neuroradiol. 2006. Vol. 27, № 2. P. 452–454.
DiPoce J., Guelfguat M., DiPoce J. Radiologic Findings in Cases of Attempted Suicide and Other Self-Injurious Behavior // RadioGraphics. 2012. Vol. 32, № 7. P. 2005–2024.
Sun Q. et al. Clinical Characteristics of Methanol-Induced Optic Neuropathy: Correlation between Aetiology and Clinical Findings // J. Ophthalmol. 2022. Vol. 2022. P. 1–11.
Ikeda M., Tsukagoshi H. Encephalopathy due to Toluene Sniffing // Eur. Neurol. 1990. Vol. 30, № 6. P. 347–349.
Aydin K. et al. Cranial MR findings in chronic toluene abuse by inhalation. // AJNR. Am. J. Neuroradiol. 2002. Vol. 23, № 7. P. 1173–1179.
Lo C.-P. et al. Brain Injury After Acute Carbon Monoxide Poisoning: Early and Late Complications // Am. J. Roentgenol. 2007. Vol. 189, № 4. P. W205–W211.
Silver D.A.T. et al. Computed tomography of the brain in acute carbon monoxide poisoning // Clin. Radiol. 1996. Vol. 51, № 7. P. 480–483.
O’DONNELL P. et al. The Magnetic Resonance Imaging Appearances of the Brain in Acute Carbon Monoxide Poisoning // Clin. Radiol. 2000. Vol. 55, № 4. P. 273–280.
Kim J. et al. Delayed encephalopathy of acute carbon monoxide intoxication: diffusivity of cerebral white matter lesions. // AJNR. Am. J. Neuroradiol. 2003. Vol. 24, № 8. P. 1592–1597.
Taheri M.S. et al. The value of brain CT findings in acute methanol toxicity // Eur. J. Radiol. 2010. Vol. 73, № 2. P. 211–214.
Kuriyama A. et al. Metronidazole-Induced Central Nervous System Toxicity // Clin. Neuropharmacol. 2011. Vol. 34, № 6. P. 241–247.
Kim E. et al. MR Imaging of Metronidazole-Induced Encephalopathy: Lesion Distribution and Diffusion-Weighted Imaging Findings // Am. J. Neuroradiol. 2007. Vol. 28, № 9. P. 1652–1658.
Kumar G., Goyal M.K. Lentiform Fork sign: A unique MRI picture. Is metabolic acidosis responsible? // Clin. Neurol. Neurosurg. 2010. Vol. 112, № 9. P. 805–812.
Grasso D. et al. Lentiform Fork Sign: A Magnetic Resonance Finding in a Case of Acute Metabolic Acidosis // Neuroradiol. J. 2014. Vol. 27, № 3. P. 288–292.
Lim C.G., Hahm M.H., Lee H.J. Hepatic encephalopathy on magnetic resonance imaging and its uncertain differential diagnoses: a narrative review // J. Yeungnam Med. Sci. 2023. Vol. 40, № 2. P. 136–145.
Geibprasert S., Gallucci M., Krings T. Alcohol-induced changes in the brain as assessed by MRI and CT // Eur. Radiol. 2010. Vol. 20, № 6. P. 1492–1501.
Zhou Y. et al. Hemichorea in nonketotic hyperglycemia: Putamenal and cerebellum lesion on MR imaging // World J. Neurosci. 2012. Vol. 02, № 02. P. 138–140.
Wintermark M. et al. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. // AJNR. Am. J. Neuroradiol. 2004. Vol. 25, № 6. P. 975–976.
Lai P.H. et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. // AJNR. Am. J. Neuroradiol. 1996. Vol. 17, № 6. P. 1057–1064.
Gambini A. et al. Marchiafava-Bignami disease: longitudinal MR imaging and MR spectroscopy study. // AJNR. Am. J. Neuroradiol. 2003. Vol. 24, № 2. P. 249–253.
Bourekas E.C. et al. Lesions of the Corpus Callosum: MR Imaging and Differential Considerations in Adults and Children // Am. J. Roentgenol. 2002. Vol. 179, № 1. P. 251–257.
Filley C.M., Kleinschmidt-DeMasters B.K. Toxic Leukoencephalopathy // N. Engl. J. Med. 2001. Vol. 345, № 6. P. 425–432.
McKinney A.M. et al. Acute Toxic Leukoencephalopathy: Potential for Reversibility Clinically and on MRI With Diffusion-Weighted and FLAIR Imaging // Am. J. Roentgenol. 2009. Vol. 193, № 1. P. 192–206.
Sindhwani G. MRI in Chemotherapy induced Leukoencephalopathy: Report of Two Cases and Radiologist’s Perspective // J. Clin. DIAGNOSTIC Res. 2017.
YAN R. et al. Clinical features and magnetic resonance image analysis of 15 cases of demyelinating leukoencephalopathy induced by levamisole // Exp. Ther. Med. 2013. Vol. 6, № 1. P. 71–74.
Lucia P. et al. Multifocal leucoencephalopathy induced by levamisole // Lancet. 1996. Vol. 348, № 9039. P. 1450.
Kass-Hout T. et al. “Chasing the dragon”--heroin-associated spongiform leukoencephalopathy. // J. Med. Toxicol. 2011. Vol. 7, № 3. P. 240–242.
Offiah C., Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy // Clin. Radiol. 2008. Vol. 63, № 2. P. 146–152.
Kriegstein A.R. et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”) // Neurology. 1999. Vol. 53, № 8. P. 1765–1765.
Howard S.A. et al. Osmotic Demyelination Syndrome // RadioGraphics. 2009. Vol. 29, № 3. P. 933–938.
Biotti D., Durupt S. A trident in the brain, central pontine myelinolysis: Figure // Pract. Neurol. 2009. Vol. 9, № 4. P. 231–232.
307. Juergenson I. et al. Teaching Neuro Images : Neuroradiologic findings in pontine and extrapontine myelinolysis // Neurology. 2012. Vol. 78, № 1.
Graff-Radford J. et al. Clinical and Radiologic Correlations of Central Pontine Myelinolysis Syndrome // Mayo Clin. Proc. 2011. Vol. 86, № 11. P. 1063–1067.
Ruzek K.A., Campeau N.G., Miller G.M. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. // AJNR. Am. J. Neuroradiol. 2004. Vol. 25, № 2. P. 210–213.
Скворцова В.И., Губский Л.В., Мельникова Е.А. Синдром задней обратимой энцефалопатии // Журнал неврологии и психиатрии им. С.С. Корсакова. 2010. Vol. 110, № 5. P. 104–109.
Bartynski W.S., Boardman J.F. Distinct Imaging Patterns and Lesion Distribution in Posterior Reversible Encephalopathy Syndrome // Am. J. Neuroradiol. 2007. Vol. 28, № 7. P. 1320–1327.
Bartynski W.S. et al. Posterior Reversible Encephalopathy Syndrome after Solid Organ Transplantation // Am. J. Neuroradiol. 2008. Vol. 29, № 5. P. 924–930.
Bartynski W.S. Posterior Reversible Encephalopathy Syndrome, Part 1: Fundamental Imaging and Clinical Features // Am. J. Neuroradiol. 2008. Vol. 29, № 6. P. 1036–1042.
Tetsuka S., Ogawa T. Posterior reversible encephalopathy syndrome: A review with emphasis on neuroimaging characteristics // J. Neurol. Sci. 2019. Vol. 404. P. 72–79.
Maier S. et al. Central-variant posterior reversible encephalopathy syndrome in a young patient with systemic lupus erythematosus // Acta Neurol. Belg. 2019. Vol. 119, № 2. P. 269–271.
Karia S.J. et al. Utility and Significance of Gadolinium-Based Contrast Enhancement in Posterior Reversible Encephalopathy Syndrome // Am. J. Neuroradiol. 2016. Vol. 37, № 3. P. 415–422.
Starkey J. et al. Cytotoxic Lesions of the Corpus Callosum That Show Restricted Diffusion: Mechanisms, Causes, and Manifestations // RadioGraphics. 2017. Vol. 37, № 2. P. 562–576.
Malhotra H. et al. Boomerang sign: Clinical significance of transient lesion in splenium of corpus callosum // Ann. Indian Acad. Neurol. 2012. Vol. 15, № 2. P. 151.
Wolfsdorf J. Diabetic Ketoacidosis in Infants, Children, and Adolescents: A consensus statement from the American Diabetes Association // Diabetes Care. 2006. Vol. 29, № 5. P. 1150–1159.
Klingensmith G.J. et al. Diabetic Ketoacidosis at Diabetes Onset: Still an All Too Common Threat in Youth // J. Pediatr. 2013. Vol. 162, № 2. P. 330-334.e1.
Dabelea D. et al. Trends in the Prevalence of Ketoacidosis at Diabetes Diagnosis: The SEARCH for Diabetes in Youth Study // Pediatrics. 2014. Vol. 133, № 4. P. e938–e945.
Edge J.A. et al. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis // Arch. Dis. Child. 2001. Vol. 85, № 1. P. 16–22.
Levin D.L. Cerebral edema in diabetic ketoacidosis // Pediatr. Crit. Care Med. 2008. Vol. 9, № 3. P. 320–329.
Barrot A., Huisman T.A., Poretti A. Neuroimaging findings in acute pediatric diabetic ketoacidosis // Neuroradiol. J. 2016. Vol. 29, № 5. P. 317–322.
Matsuura H., Nakamura T. Inverted V sign: subacute combined degeneration of the spinal cord // QJM An Int. J. Med. 2018. Vol. 111, № 1. P. 65–66.
Narra R. “Inverted V sign” in Sub-Acute Combined Degeneration of Cord // J. Clin. DIAGNOSTIC Res. 2015.
Naidich M.J., Ho S.U. Case 87: Subacute Combined Degeneration // Radiology. 2005. Vol. 237, № 1. P. 101–105.
Kumar A., Singh A.K. Teaching Neuro Image : Inverted V sign in subacute combined degeneration of spinal cord // Neurology. 2009. Vol. 72, № 1.
Karantanas A.H., Markonis A., Bisbiyiannis G. Subacute combined degeneration of the spinal cord with involvement of the anterior columns: a new MRI finding // Neuroradiology. 2000. Vol. 42, № 2. P. 115–117.
Holroyd K.B., Berkowitz A.L. Metabolic and Toxic Myelopathies // Contin. Lifelong Learn. Neurol. 2024. Vol. 30, № 1. P. 199–223.
Ravina B., Loevner L.A., Bank W. MR Findings in Subacute Combined Degeneration of the Spinal Cord // Am. J. Roentgenol. 2000. Vol. 174, № 3. P. 863–865.
Kuker W. et al. MRI demonstration of reversible impairment of the blood-CNS barrier function in subacute combined degeneration of the spinal cord. // J. Neurol. Neurosurg. Psychiatry. 1997. Vol. 62, № 3. P. 298–299.
Rocha E.A. et al. RCVS 2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome // Neurology. 2019. Vol. 92, № 7.
Miller T.R. et al. Reversible Cerebral Vasoconstriction Syndrome, Part 2: Diagnostic Work-Up, Imaging Evaluation, and Differential Diagnosis // Am. J. Neuroradiol. 2015. Vol. 36, № 9. P. 1580–1588.
Singhal A.B. Reversible Cerebral Vasoconstriction Syndromes // Arch. Neurol. 2011. Vol. 68, № 8. P. 1005.
Chen S. et al. Transcranial color doppler study for reversible cerebral vasoconstriction syndromes // Ann. Neurol. 2008. Vol. 63, № 6. P. 751–757.
Bogousslavsky J. et al. Postpartum Cerebral Angiopathy: Reversible Vasoconstriction Assessed by Transcranial Doppler Ultrasounds // Eur. Neurol. 1989. Vol. 29, № 2. P. 102–105.
Mandell D.M. et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology // Am. J. Neuroradiol. 2017. Vol. 38, № 2. P. 218–229.
Perillo T. et al. Reversible cerebral vasoconstriction syndrome: review of neuroimaging findings // Radiol. Med. 2022. Vol. 127, № 9. P. 981–990.
340. Huang B.Y., Castillo M. Hypoxic-Ischemic Brain Injury: Imaging Findings from Birth to Adulthood // RadioGraphics. 2008. Vol. 28, № 2. P. 417–439.
Han B.K. et al. Reversal sign on CT: effect of anoxic/ischemic cerebral injury in children. // AJNR. Am. J. Neuroradiol. 1989. Vol. 10, № 6. P. 1191–1198.
Muttikkal T.J.E., Wintermark M. MRI patterns of global hypoxic-ischemic injury in adults // J. Neuroradiol. 2013. Vol. 40, № 3. P. 164–171.
Yuzawa H. et al. Pseudo-Subarachnoid Hemorrhage Found in Patients with Postresuscitation Encephalopathy: Characteristics of CT Findings and Clinical Importance // Am. J. Neuroradiol. 2008. Vol. 29, № 8. P. 1544–1549.
Sawada H. et al. MRI demonstration of cortical laminar necrosis and delayed white matter injury in anoxic encephalopathy // Neuroradiology. 1990. Vol. 32, № 4. P. 319–321.
Allen L.M. et al. Sequence-specific MR Imaging Findings That Are Useful in Dating Ischemic Stroke // RadioGraphics. 2012. Vol. 32, № 5. P. 1285–1297.
Given C.A. et al. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. // AJNR. Am. J. Neuroradiol. 2003. Vol. 24, № 2. P. 254–256.
Anne G. Osborn. Osborns Brain: Imaging, Pathology, and Anatomy. Amirsys, 2013. Vol. 1272.
Zamora C.A. et al. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature // Brain Behav. 2015. Vol. 5, № 8.
Beeskow A.B. et al. Delayed Post-hypoxic Leukoencephalopathy (DPHL)—An Uncommon Variant of Hypoxic Brain Damage in Adults // Front. Neurol. 2018. Vol. 9.
Shprecher D., Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. // NeuroRehabilitation. 2010. Vol. 26, № 1. P. 65–72.
Misra U.K. et al. Spectrum of hyperosmolar hyperglycaemic state in neurology practice // Indian J. Med. Res. 2017. Vol. 146, № Suppl 2. P. S1–S7.
Marren S.M., Beale A., Yiin G.S. Hyperosmolar hyperglycaemic state as a stroke cause or stroke mimic: an illustrative case and review of literature // Clin. Med. (Northfield. Il). 2022. Vol. 22, № 1. P. 83–86.
Bala M.I. et al. Teaching NeuroImages: Nonketotic hyperglycemic hyperosmolar state mimicking acute ischemic stroke // Neurology. 2020. Vol. 95, № 18.
Клинические рекомендации. Эпилепсия и эпилептический статус у взрослых и детей. 2022. Vol. 277 с.
Beghi E. et al. Recommendation for a definition of acute symptomatic seizure // Epilepsia. 2010. Vol. 51, № 4. P. 671–675.
Edlow J.A. et al. Diagnosis of reversible causes of coma // Lancet. 2014. Vol. 384, № 9959. P. 2064–2076.
Halavaara J. et al. Wernicke’s encephalopathy: is diffusion-weighted MRI useful? // Neuroradiology. 2003. Vol. 45, № 8. P. 519–523.
Weidauer S. et al. Wernicke encephalopathy: MR findings and clinical presentation // Eur. Radiol. 2003. Vol. 13, № 5. P. 1001–1009.
Jung Y.-C., Chanraud S., Sullivan E. V. Neuroimaging of Wernicke’s Encephalopathy and Korsakoff’s Syndrome // Neuropsychol. Rev. 2012. Vol. 22, № 2. P. 170–180.
Van Berkel B. et al. Subacute combined degeneration of the cervical and dorsal spinal cord in a 40-year-old male patient: A case report // Radiol. Case Reports. 2021. Vol. 16, № 1. P. 13–17.
Desai A.B. et al. Motor Neuron Diseases and Central Nervous System Tractopathies: Clinical-Radiologic Correlation and Diagnostic Approach // RadioGraphics. 2025. Vol. 45, № 1.
Leitinger M. et al. Salzburg Consensus Criteria for Non-Convulsive Status Epilepticus – approach to clinical application // Epilepsy Behav. 2015. Vol. 49. P. 158–163.
Mauritz M. et al. Acute symptomatic seizures: an educational, evidence‐based review // Epileptic Disord. 2022. Vol. 24, № 1. P. 26–49.
Пирадов М.А. et al. Электронейромиография: алгоритмы и рекомендации при полинейропатиях. Москва: “Горячая линия - Телеком,” 2021. Vol. 198 с.
Общероссийская общественная организация «Ассоциация врачей-офтальмологов». Клинические рекомендации: Сахарный диабет: диабетическая ретинопатия, диабетический макулярный отек. 2022.
Общероссийская общественная организация «Ассоциация врачей-офтальмологов», Общероссийская общественная организация «Общество офтальмологов России». Клинические рекомендации: Эндокринная офтальмопатия при аутоиммунной патологии щитовидной железы у взрослых . 2024.
Офтальмология. Национальное руководство / ed. под ред. С.Э. Аветисова Е.А.Е.Л.К.М.В.В.Н.Х.П.Т. Москва: ГЭОТАР-Медиа, 2024. Vol. 952.
Wijnia J.W. A Clinician’s View of Wernicke-Korsakoff Syndrome // J. Clin. Med. 2022. Vol. 11, № 22. P. 6755.
Wu H. et al. Assessment of rehabilitation treatment for patients with acute poisoning-induced toxic encephalopathy. // World J. Emerg. Med. 2024. Vol. 15, № 6. P. 441–447.
Dedov I. et al. Standards of Specialized Diabetes Care / Edited by Dedov I.I., Shestakova M.V., Mayorov A.Yu. 11th Edition // Diabetes Mellit. 2023. Vol. 26, № 2S. P. 1–157.
Медицинская реабилитация руководство в 3-х томах / ed. под ред. Боголюбова В.М. Москва: Бином, 2010. Vol. 416.
Buylaert W.A. Coma induced by intoxication. // Acta Neurol. Belg. 2000. Vol. 100, № 4. P. 221–224.
Bartlett D. The Coma Cocktail: Indications, Contraindications, Adverse Effects, Proper Dose, and Proper Route // J. Emerg. Nurs. 2004. Vol. 30, № 6. P. 572–574.
Sivilotti M.L.A. Flumazenil, naloxone and the ‘coma cocktail’ // Br. J. Clin. Pharmacol. 2016. Vol. 81, № 3. P. 428–436.
Shikalova I.A. et al. Results of a Multicenter Study on the Efficacy and Safety of Inosine Glycyl-Cysteinyl-Glutamate Disodium in the Treatment of Acute Ethanol Poisoning // Russ. Sklifosovsky J. “Emergency Med. Care.” 2022. Vol. 11, № 3. P. 444–456.
Aleksandrov M. V. et al. Effect of reconstituted glutathione preparations on bioelectric brain activity in severe ethanol poisoning // Toxicol. Rev. 2020. № 2. P. 18–24.
Антушевич А.Е. et al. Оценка эффективности применения инозина глицил-цистеинил-глутамата динатрия при острых тяжелых отравлениях этанолом // Вестник Российской Военно-медицинской академии. 2017. Vol. 2, № 58. P. 7–12.
Бузанов Д.В. et al. Применение моликсана для раннего лечения алкогольной комы // Скорая медицинская помощь. 2016. Vol. 17, № 4. P. 70–75.
Das S. et al. Management of Cerebral Herniation Secondary to Lead Encephalopathy: A Case Report // Front. Neurol. 2022. Vol. 13.
Raut T.P. et al. Acute Lead Encephalopathy Secondary to Ayurvedic Medication Use // Neurol. India. 2021. Vol. 69, № 5. P. 1417–1420.
Whitfield C.L., Ch’ien L.T., Whitehead J.D. Lead encephalopathy in adults // Am. J. Med. 1972. Vol. 52, № 3. P. 289–298.
Dhaliwal J.S., Rosani A., Saadabadi A. Diazepam. 2025.
Kim H.K. et al. Safety and efficacy of pharmacologic agents used for rapid tranquilization of emergency department patients with acute agitation or excited delirium // Expert Opin. Drug Saf. 2021. Vol. 20, № 2. P. 123–138.
Оказание медицинской помощи больным с острыми отравлениями на догоспитальном и раннем госпитальном этапах / ed. под ред. Миннуллина И.П. Санкт-Петербург: Первый Санкт-Петербургский государственный медицинский университет им. акад. И.П. Павлова, НИИ скорой помощи им. И.И. Джанелидзе, 2018.
Шилов В.В., Васильев С.А., Кузнецов О.А. Клинические рекомендации (протоколы) по оказанию скорой медицинской помощи при острых отравлениях. 2014. Vol. 28.
Багненко С.Ф. Скорая медицинская помощь. Национальное руководство (2-е издание, переработанное и дополненное). Москва: ГЭОТАР-Медиа, 2015. Vol. 1032.
Интенсивная терапия. Национальное руководство. Краткое издание, 2-е изд., перераб. и доп. / ed. под ред. Б.Р. Гельфанда И.Б.З. Москва: ГЭОТАР-Медиа, 2019. Vol. 928.
Girard T.D. et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness // N. Engl. J. Med. 2018. Vol. 379, № 26. P. 2506–2516.
McQueen J. et al. Brief interventions for heavy alcohol users admitted to general hospital wards // Cochrane Database Syst. Rev. 2011. Vol. 2015, № 9.
Kaner E.F. et al. Effectiveness of brief alcohol interventions in primary care populations // Cochrane Database Syst. Rev. 2018. Vol. 2018, № 6.
Curry S.J. et al. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults // JAMA. 2018. Vol. 320, № 18. P. 1899.
O’Connor E.A. et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: an updated systematic review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality, 2018.
Greene M.C. et al. Psychosocial and pharmacologic interventions to reduce harmful alcohol use in low- and middle-income countries // Cochrane Database Syst. Rev. 2023. Vol. 2023, № 5.
Dingwall K.M. et al. What is the optimum thiamine dose to treat or prevent Wernicke’s encephalopathy or Wernicke–Korsakoff syndrome? Results of a randomized controlled trial // Alcohol. Clin. Exp. Res. 2022. Vol. 46, № 6. P. 1133–1147.
Шавловская О.А. Алкогольные поражения нервной системы. Нейропротективная терапия // Эффект. фармакотер. . 2014. Vol. 17. P. 16–24.
Шавловская О.А. Возможности терапии посталкогольных изменений нервной системы // Лечащий врач. 2014. Vol. 5. P. 24.
Игнатова Т.В. Цераксон в коррекции когнитивных нарушений при хронической алкогольной интоксикации в сочетании с другими заболеваниями головного мозга // Справочник терапевтического врача. 2014. P. 14–17.
Шавловская О.А. Цитиколин: новые терапевтические возможности // Лечащий врач. 2014. Vol. 10. P. 29–33.
Ilyinsky N.S. et al. The influence of cytoflavin on the recovery of cognitive function in elderly people with alcohol abuse // Zhurnal Nevrol. i psikhiatrii im. S.S. Korsakova. 2016. Vol. 116, № 11. Vyp. 2. P. 49.
Соловьев А.Г., Елистратова Т.В. Эффективность кортексина в комплексной терапии больных с хронической алькогольной энцефалопатией и полиневропатией // Журнал Неврологии и психитарии им. С.С. Корсакова. 2010. Vol. 4. P. 48–51.
Potupchik T. V., Veselova O.F., Gatskikh I. V. Pharmacotherapeutic aspects of nootropics use in people with alcohol dependence // Med. Alph. 2019. Vol. 2, № 19. P. 37–41.
Vostrikov V. V. Place of piracetam in the modern practice of medicine // Rev. Clin. Pharmacol. Drug Ther. 2017. Vol. 15, № 1. P. 14–25.
Potupchik T., Lopatina T., Lopatin V. Nootropic drugs in the combination therapy of chronic alcoholism // Vrach. 2018. Vol. 29, № 11.
Казакова Ю.А., Карпов С.М., Шевченко П.П. Алкогольная энцефалопатия: современные методы лечения // Успехи современного естествознания. 2014. Vol. 6. P. 22–23.
Лопатин В., Лопатина Т. Применение глицина при лечении алкоголизма // Врач. 2017. Vol. 28, № 7. P. 41–42.
Dharavath R.N. et al. GABAergic signaling in alcohol use disorder and withdrawal: pathological involvement and therapeutic potential // Front. Neural Circuits. 2023. Vol. 17.
Patel S., Topiwala K., Hudson L. Wernicke’s Encephalopathy // Cureus. 2018.
Trukhanova I.G., Zinatullina D.S., Gureev A.D. Possibilities of Application of Ethylmethylhydroxypyri dine Succinate in the Treatment of Acute Poisoning: a Systematic Literature Review // Russ. Sklifosovsky J. “Emergency Med. Care.” 2024. Vol. 13, № 2. P. 280–287.
Vasil’ev S.A. et al. Acute Intoxications Involving Synthetic Psychoactive Substances // Gen. Reanimatol. 2018. Vol. 14, № 1. P. 23–28.
Почепень О.Н. Оценка эффективности цитофлавина при лечении токсико-гипоксической энцефалопатии после тяжелой термической травмы // Журнал неврологии и психиатрии им. С.С. Корсакова. . 2010. Vol. 110, № 10. P. 23–29.
Ливанов Г.А. et al. Фармакологическая коррекция токсико-гипоксической энцефалопатии у больных с тяжелыми формами острых отравлений // Вестник экстренной медицины. 2017. Vol. 11, № 3. P. 51–54.
Vasilyev S. et al. [Use of cytoflavin into the complex intensive therapy of acute cerebral insufficiency caused by poisoning]. // Georgian Med. News. 2012. № 203. P. 22–29.
Sinenchenko A.G. et al. Acute severe oral poisoning with 1.4-butandiol and ethanol with the development of coma // Zhurnal Nevrol. i psikhiatrii im. S.S. Korsakova. 2020. Vol. 120, № 3. P. 77.
Okovityĭ S. V et al. [Antihypoxants in current clinical practice]. // Klin. Med. (Mosk). 2012. Vol. 90, № 9. P. 63–68.
Cho S., Lee M.J., Chung C.-S. Effect of Nimodipine Treatment on the Clinical Course of Reversible Cerebral Vasoconstriction Syndrome // Front. Neurol. 2019. Vol. 10.
Kurushina O. V., Barulin A.E., Chernovolenko E.P. Alcoholic polyneuropathy: ways of diagnostics and therapy // Med. Counc. 2019. № 1. P. 58–63.
Емельянова А.Ю., Зиновьева О.Е. Алкогольная полиневропатия: клинико-патогенетические варианты, принципы диагностики и лечения // Эффективная факмакотерапия. 2015. Vol. 2, № 13.
Кугелева А.О. Актовегин в комплексном лечении полиневропатий // Вестник Смоленской государственной медицинской академии. 2002. Vol. 3. P. 32–34.
Gusev V. V., Zaitseva O. V., Lvova O.A. Effectiveness of gabapentin in treatment of pain in patients with alcoholic polyneuropathy // Med. Alph. 2024. № 33. P. 20–24.
Chopra K., Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities // Br. J. Clin. Pharmacol. 2012. Vol. 73, № 3. P. 348–362.
Шамалов Н. et al. Диабетическая и алкогольная полинейропатии // Врач. 2015. Vol. 11. P. 13–15.
Loprinzi C.L. et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update // J. Clin. Oncol. 2020. Vol. 38, № 28. P. 3325–3348.
Bangert M.K., Hasbun R. Neurological and Psychiatric Adverse Effects of Antimicrobials // CNS Drugs. 2019. Vol. 33, № 8. P. 727–753.
Guo Y. et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial // Support. Care Cancer. 2014. Vol. 22, № 5. P. 1223–1231.
Holotiuk I.S. et al. EFFECTIVENESS OF ALPHA-LIPOIC ACID AND IPIDACRINE HYDROCHLORIDE IN PREVENTION OF PACLITAXEL-INDUCED PERIPHERAL NEUROPATHY ASSESSED BY ELECTRONEUROMYOGRAPHY OF SUPERFICIAL PERONEAL AND SURAL NERVES // Exp. Oncol. 2023. Vol. 44, № 4. P. 300–306.
Pizova N. V. Main metabolic and toxic polyneuropathies in clinical practice // Meditsinskiy Sov. = Med. Counc. 2021. № 19. P. 134–146.
Levin O.S., Matvievskaya O. V. Evaluation of epidemiological data on effect of therapy with Ipigrix® on motor and sensory functions in ambulatory patients with various diseases ofperipheral nervous system // Med. Alph. 2019. Vol. 1, № 2. P. 11–14.
Derry S. et al. Topical lidocaine for neuropathic pain in adults // Cochrane Database of Systematic Reviews / ed. Derry S. Chichester, UK: John Wiley & Sons, Ltd, 2014.
Ozdemir D. et al. Topical menthol for chemotherapy-induced peripheral neuropathy: a randomised controlled trial in breast cancer // BMJ Support. Palliat. Care. 2025. Vol. 15, № 1. P. 79–86.
Wolf S. et al. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies // Eur. J. Cancer. 2008. Vol. 44, № 11. P. 1507–1515.
Tkachenko E.V. et al. Neurotoxicity as a side effect of taxanes in cancer patients // Russ. J. Pain. 2020. Vol. 18, № 3. P. 48.
Латипова Д.Х. et al. Неврологические осложнения противоопухолевой лекарственной терапии // Malig. tumours. 2023. Vol. 13, № 3s2-2. P. 302–311.
Cavaletti G., Zanna C. Current status and future prospects for the treatment of chemotherapy-induced peripheral neurotoxicity // Eur. J. Cancer. 2002. Vol. 38, № 14. P. 1832–1837.
Smith E.M.L. et al. Effect of Duloxetine on Pain, Function, and Quality of Life Among Patients With Chemotherapy-Induced Painful Peripheral Neuropathy // JAMA. 2013. Vol. 309, № 13. P. 1359.
Jordan B. et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up // Ann. Oncol. 2020. Vol. 31, № 10. P. 1306–1319.
Farshchian N. et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy // Cancer Chemother. Pharmacol. 2018. Vol. 82, № 5. P. 787–793.
Saarto T., Wiffen P.J. Antidepressants for neuropathic pain // Cochrane Database Syst. Rev. 2007. Vol. 2014, № 1.
Catalisano G. et al. Neuropathic pain, antidepressant drugs, and inflammation: a narrative review // J. Anesth. Analg. Crit. Care. 2024. Vol. 4, № 1. P. 67.
Moore R.A. et al. Amitriptyline for neuropathic pain in adults // Cochrane Database Syst. Rev. 2015. Vol. 2019, № 5.
Caraceni A. et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC // Lancet Oncol. 2012. Vol. 13, № 2. P. e58–e68.
Fallon M., Hanks G., Cherny N. Principles of control of cancer pain // BMJ. 2006. Vol. 332, № 7548. P. 1022–1024.
Kurz A., Sessler D.I. Opioid-Induced Bowel Dysfunction // Drugs. 2003. Vol. 63, № 7. P. 649–671.
Соловьева Э.Ю. et al. Клинические рекомендации по применению нейротропных витаминов группы В (В1, В6 и В12) для лечения периферической невропатии: консенсус многопрофильной экспертной группы // Нервные болезни. 2024. Vol. 1. P. 91–98.
Viana M.D.M. et al. Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain. // Antioxidants (Basel, Switzerland). 2022. Vol. 11, № 12.
Finnerup N.B. et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis // Lancet Neurol. 2015. Vol. 14, № 2. P. 162–173.
Baron R., Mahn F. Topische Therapieformen bei peripheren neuropathischen Schmerzen // Der Schmerz. 2010. Vol. 24, № 4. P. 317–325.
Wiffen P.J. et al. Gabapentin for chronic neuropathic pain in adults // Cochrane Database Syst. Rev. 2017. Vol. 2020, № 2.
Derry S. et al. Pregabalin for neuropathic pain in adults // Cochrane Database Syst. Rev. 2019.
Bockbrader H.N. et al. A Comparison of the Pharmacokinetics and Pharmacodynamics of Pregabalin and Gabapentin // Clin. Pharmacokinet. 2010. Vol. 49, № 10. P. 661–669.
Patel R., Dickenson A.H. Mechanisms of the gabapentinoids and α 2 δ ‐1 calcium channel subunit in neuropathic pain // Pharmacol. Res. Perspect. 2016. Vol. 4, № 2.
Zhou M. et al. Oxcarbazepine for neuropathic pain // Cochrane Database Syst. Rev. 2017. Vol. 2017, № 12.
Lunn M.P., Hughes R.A., Wiffen P.J. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia // Cochrane Database Syst. Rev. 2014. Vol. 2015, № 10.
Birkinshaw H. et al. Antidepressants for pain management in adults with chronic pain: a network meta-analysis // Cochrane Database Syst. Rev. 2023. Vol. 2023, № 5.
Gallagher H.C. et al. Venlafaxine for neuropathic pain in adults // Cochrane Database Syst. Rev. 2015. Vol. 2017, № 6.
Aiyer R., Barkin R.L., Bhatia A. Treatment of Neuropathic Pain with Venlafaxine: A Systematic Review // Pain Med. 2016. P. pnw261.
Boyle J. et al. Randomized, Placebo-Controlled Comparison of Amitriptyline, Duloxetine, and Pregabalin in Patients With Chronic Diabetic Peripheral Neuropathic Pain // Diabetes Care. 2012. Vol. 35, № 12. P. 2451–2458.
Freo U., Romualdi P., Kress H.G. <p>Tapentadol for neuropathic pain: a review of clinical studies</p> // J. Pain Res. 2019. Vol. Volume 12. P. 1537–1551.
Atkinson T.J. et al. Medication Pain Management in the Elderly: Unique and Underutilized Analgesic Treatment Options // Clin. Ther. 2013. Vol. 35, № 11. P. 1669–1689.
Gaskell H. et al. Oxycodone for neuropathic pain in adults // Cochrane Database Syst. Rev. 2016. Vol. 2016, № 7.
Duehmke R.M. et al. Tramadol for neuropathic pain in adults // Cochrane Database Syst. Rev. 2017. Vol. 2017, № 6.
Cooper T.E. et al. Morphine for chronic neuropathic pain in adults // Cochrane Database Syst. Rev. 2017. Vol. 2019, № 5.
Nucynta E.R. (tapentadol HCl) [package insert]. http://www.ac cessdata.fda.gov/drugsatfda_ docs/label/2012/200533s001lbl. pdf. Accessed August 14, 2013 [Electronic resource].
Karateev A.E. et al. RATIONAL USE OF NONSTEROIDAL ANTI-INFLAMMATORY DRUGS. CLINICAL GUIDELINES // Rheumatol. Sci. Pract. 2018. Vol. 56. P. 1–29.
Усманова Д.Д., Хажибакиев Х.Х. Эффективность берлитиона в лечении диабетической энцефалопатии // Доброхотовские чтения. Материалы II междисциплинарной научной конференции, посвященной 85-летию Дагестанского государственного медицинского университета. 2017. P. 159–160.
Мохорт Т.В. Когнитивная функция и сахарный диабет: что должен знать клиницист? // Медицинские новости. 2018. Vol. 10, № 289.
Zhuravleva M. V. et al. Meta-analytical evaluation of the clinical efficacy of a complex metabolic neuroprotector in patients with chronic cerebral ischemia // Antibiot. Chemother. 2022. Vol. 66, № 9–10. P. 39–53.
Gatckikh I. V. et al. Dynamics of cognitive disorders in patients with type 2 diabetes mellitus under effect of metabolic therapy // Clin. Med. (Russian Journal). 2016. Vol. 94, № 7. P. 533–539.
Шабалина Н.И. Актовегин в лечении дисциркуляторной энцефалопатии и диабетической полинейропатии // Нервные болезни. 2008. Vol. 4. P. 10–12.
Голубев А.Д., Зиньковская Т.М., Барламов П.Н. Влияние нейропротекции на функции центральной, периферической нервной систем и некоторые гемодинамические показатели у больных сахарным диабетом // Пермский медицинский журнал. 2012. Vol. 29, № 3. P. 36–41.
Чуйко М.Р., Ефремова Н.М., Скворцова В.И. Эффективность и безопасность применения глицина и лимонтара в комплексной терапии дисциркуляторной энцефалопатии и энцефалопатии при инсулинзависимом сахарном диабете // Журнал неврологии и психиатрии им. С.С. Корсакова. 2010. Vol. 110, № 6. P. 44–48.
Ang L. et al. Glucose Control and Diabetic Neuropathy: Lessons from Recent Large Clinical Trials // Curr. Diab. Rep. 2014. Vol. 14, № 9. P. 528.
Balducci S. et al. Exercise training can modify the natural history of diabetic peripheral neuropathy // J. Diabetes Complications. 2006. Vol. 20, № 4. P. 216–223.
Gibbons C.H., Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes // Brain. 2015. Vol. 138, № 1. P. 43–52.
Trukhan D.I., Druk I. V. Effectiveness of use of group B vitamins in patients with diabetic polyneuropathy: A review // Cons. Medicum. 2024. Vol. 26, № 4. P. 269–275.
Хасанова Э.Р., Петунина Н.А., Галстян К.О. Возможности нейропротекторной терапии в лечении диабетической полинейропатии // Эффективная фармакотерапия. Эндокринология. 2011. Vol. 3. P. 2–7.
Ziegler D. et al. Current concepts in the management of diabetic polyneuropathy. // J. Diabetes Investig. 2021. Vol. 12, № 4. P. 464–475.
Hsieh R.-Y. et al. Effects of Oral Alpha-Lipoic Acid Treatment on Diabetic Polyneuropathy: A Meta-Analysis and Systematic Review. // Nutrients. 2023. Vol. 15, № 16.
Солуянова Т.Н. Альфа-липоевая кислота в лечении диабетической полинейропатии с позиций доказательной медицины // Эндокринология: новости, мнения, обучение. 2018. Vol. 7, № 4. P. 48–53.
Аленикова О.А. Features of Clinical Manifestations of Diabetic Polyneuropathies and their Treatment // Рецепт. 2024. Vol. 27, № 3. P. 390–402.
Низовцева О.А. Современные аспекты лечения диабетической полинейропатии // Трудный пациент. 2019. Vol. 17, № 5. P. 15–18.
Rachin A.P. et al. Diabetic Polyneuropathy: from Pathogenesis to Therapy and Prevention (to Help the General Practitioner) // Comorbidity Neurol. 2024. Vol. 1, № 3. P. 101–108.
Shchepankevich L.A. et al. Painful diabetic polyneuropathy: focus on life quality improvement of patients // Zhurnal Nevrol. i psikhiatrii im. S.S. Korsakova. 2019. Vol. 119, № 5. P. 76.
Pashkova I.N. et al. Evaluation of the effectiveness of metabolic therapy in the treatment of diabetic polyneuropathies in patients with type 2 diabetes // Probl. Biol. Med. Pharm. Chem. 2020. Vol. 23, № 10. P. 25–34.
Горшков И.П., Волынкина А.П., Золоедов В.И. Опыт применения цитофлавина в лечении больных сахарным диабетом 2-го типа с диабетической полинейропатией // Проблемы Эндокринологии. 2012. Vol. 58, № 4. P. 14–15.
Dan Ziegler. Современные принципы ведения больных с диабетической полинейропатией // Нервно-мышечные болезни. 2012. Vol. 2. P. 7–19.
Galstyan G.R. et al. Diagnosis and rational treatment of painful diabetic peripheral neuropathy: an interdisciplinary expert consensus // Diabetes Mellit. 2019. Vol. 22, № 4. P. 305–327.
Савустьяненко А.В. Применение карбамазепина для лечения нейропатической боли: обзор исследований // Международный неврологический журнал. 2011. Vol. 2. P. 107–115.
Турбина Л.Г., Гордеев С.А., Зусьман А.А. Применение антидепрессантов разных фармакологических групп в комплексной терапии болевой формы диабетической полинейропатии // Сахарный диабет. 2012. Vol. 3. P. 67–73.
Khramilin V.N., Demidova I.Y. Diagnosis and Treatment of Diabetic Polyneuropathy in Elderly Patients // Eff. Pharmacother. 2020. Vol. 16, № 12. P. 42–54.
Rowbotham M.C. et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. // Pain. 2004. Vol. 110, № 3. P. 697–706.
Морозов А.М. et al. Диабетическая дистальная полинейропатия: профилактика, лечение и реабилитация (обзор литературы) // Вестник медицинского института «Реавиз» реабилитация, врач и здоровье. 2022. Vol. 3, № 57. P. 68–77.
Mel’nikova O.G. et al. Rezul’taty primeneniya strukturirovannoy programmy obucheniya bol’nykh sakharnym diabetom 2 tipa na insulinoterapii // Diabetes Mellit. 2008. Vol. 11, № 4. P. 71–75.
Бадалян А.В. Реабилитация больных // Медицинская токсикология: нац. рук-во / ed. под ред. Лужникова Е.А. Москва: ГЭОТАР-Медиа, 2012. P. 809–817.
Бадалян А.В. Реабилитация больных при острых отравлениях. Методические рекомендации № 17 / ed. Е.А. Лужников Ю.С.Г.К.К.И.А.В.Б.М.В.Б.А.Н.Е.В.А.М.А.И.Б.Е.А.Ч.Е.Е.Б.Л.И.С.И.Ю.Б.Н.С.К.М.В.Р.Т.П.П.Т.А.В.А.Ю.С.С.Б.М.Ю.А.К. Москва: НИИ СП им. Н.В. Склифосовского, 2013.
Агибалова Т.В., Нобатова В.Н., Тучина О.Д. и др. Обзор факторов, влияющих на формирование ремиссии у наркологических больных // Российский медико-биологический вестник имени академика И.П. Павлова. 2023. Vol. 31, № 1. P. 155–163.
Наркология. Национальное руководство / Н.Н. Иванец и др. 3-е изд., перераб. и доп. . Москва: ГЭОТАР-Медиа, 2024. Vol. 848.
Демина М.В. Нарушения нозогнозии (синдром отчуждения болезни) при алкоголизме и героиновой наркомании (клиника, систематика, подходы к коррекции) : диссертация ... доктора медицинских наук : 14.00.45 / Демина Мария Владимировна; [Место защиты: ФГУ “Национальный научный центр наркологии”]. — Москва, 2005. — 232 с.
Клименко Т.В., Фадеева Е.В. Типовая программа медицинской реабилитации лиц с наркологическими расстройствами: Программа. Москва: ФГБУ «НМИЦ ПН им. В.П. Сербского» Минздрава России, 2024. Vol. 80.
Iravani S. et al. Effectiveness of Acupuncture Treatment on Chemotherapy-Induced Peripheral Neuropathy: A Pilot, Randomized, Assessor-Blinded, Controlled Trial // Pain Res. Manag. 2020. Vol. 2020. P. 1–11.
Sen S., Sen S. Therapeutic effects of hyperbaric oxygen: integrated review. // Med. Gas Res. 2021. Vol. 11, № 1. P. 30–33.
Ortega M.A. et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities // Medicina (B. Aires). 2021. Vol. 57, № 9. P. 864.
Schottlender N., Gottfried I., Ashery U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. // Biomolecules. 2021. Vol. 11, № 12.
De Wolde S.D. et al. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis // Biomolecules. 2021. Vol. 11, № 8. P. 1210.
Fischer I., Barak B. Molecular and Therapeutic Aspects of Hyperbaric Oxygen Therapy in Neurological Conditions. // Biomolecules. 2020. Vol. 10, № 9.
Sutherland A.M. et al. Hyperbaric Oxygen Therapy: A New Treatment for Chronic Pain? // Pain Pract. 2016. Vol. 16, № 5. P. 620–628.
Zhou Y.-Y. et al. Advances in the treatment of neuropathic pain with hyperbaric oxygen // Undersea Hyperb. Med. 2021. Vol. 48, № 1. P. 13–23.
Cha J. et al. Hyperbaric Oxygen Therapy for Management of Complex Regional Pain Syndrome // Clin. J. Pain. 2025. Vol. 41, № 4.
Gottfried I., Schottlender N., Ashery U. Hyperbaric Oxygen Treatment—From Mechanisms to Cognitive Improvement // Biomolecules. 2021. Vol. 11, № 10. P. 1520.
Ahmadi F., Khalatbary A.R. A review on the neuroprotective effects of hyperbaric oxygen therapy. // Med. Gas Res. 2021. Vol. 11, № 2. P. 72–82.
Barata P. et al. The Role of Hyperbaric Oxygen Therapy in Neuroregeneration and Neuroprotection: A Review. // Cureus. 2024. Vol. 16, № 6. P. e62067.
Bin-Alamer O. et al. Hyperbaric oxygen therapy as a neuromodulatory technique: a review of the recent evidence. // Front. Neurol. 2024. Vol. 15. P. 1450134.
Levy R.L., Miller N.R. Hyperbaric oxygen therapy for radiation-induced optic neuropathy. // Ann. Acad. Med. Singapore. 2006. Vol. 35, № 3. P. 151–157.
Luo D. et al. Hyperbaric oxygen therapy to improve cognitive dysfunction and encephalatrophy induced by N2O for recreational use: a case report. // Neuropsychiatr. Dis. Treat. 2018. Vol. 14. P. 1963–1967.
Wang S.-H. et al. Hyperbaric Oxygen Therapy Alleviates Paclitaxel-Induced Peripheral Neuropathy Involving Suppressing TLR4-MyD88-NF-κB Signaling Pathway // Int. J. Mol. Sci. 2023. Vol. 24, № 6. P. 5379.
Bates M.E., Buckman J.F., Nguyen T.T. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. // Neuropsychol. Rev. 2013. Vol. 23, № 1. P. 27–47.
Сиволап Ю.П. Алкогольные расстройства: мишени и средства терапии // Наркология. 2014. Vol. 3, № 147. P. 34–38.
van Hout M.S.E. et al. Psychological Treatment of Patients with Chronic Toxic Encephalopathy: Lessons from Studies of Chronic Fatigue and Whiplash // Psychother. Psychosom. 2003. Vol. 72, № 5. P. 235–244.
Otis J.D. Managing Chronic Pain: Therapist Guide. Oxford University Press, 2007.
Schaper N.C. et al. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). 2023.
Sicco A. Bus I.C.. S. et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update) // Diabetes. Metab. Res. Rev. 2023.
Albers J.W., Pop-Busui R. Diabetic Neuropathy: Mechanisms, Emerging Treatments, and Subtypes // Curr. Neurol. Neurosci. Rep. 2014. Vol. 14, № 8. P. 473.
Dyck P.J. et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity // Diabetes. Metab. Res. Rev. 2011. Vol. 27, № 7. P. 620–628.
Crawford F. et al. A systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS) // Health Technol. Assess. (Rockv). 2015. Vol. 19, № 57. P. 1–210.
Dimitrova A., Murchison C., Oken B. Acupuncture for the Treatment of Peripheral Neuropathy: A Systematic Review and Meta-Analysis // J. Altern. Complement. Med. 2017. Vol. 23, № 3. P. 164–179.
Калинин А.П., Рудакова И.Г., Котов С.В. Диабетическая нейропатия // Альманах клинической медицины. 2001. Vol. 4. P. 95–107.
Hoerder S. et al. Acupuncture in diabetic peripheral neuropathy-neurological outcomes of the randomized acupuncture in diabetic peripheral neuropathy trial // World J. Diabetes. 2023. Vol. 14, № 12. P. 1813–1823.
Abuaisha B.., Costanzi J.., Boulton A.J.. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study // Diabetes Res. Clin. Pract. 1998. Vol. 39, № 2. P. 115–121.
Fan B. et al. Research trends of acupuncture therapy for painful peripheral nervous system diseases from 2004 to 2023: a bibliometric and meta-analysis // Front. Neurol. 2025. Vol. 16.
Ahn A.C. et al. Two Styles of Acupuncture for Treating Painful Diabetic Neuropathy – a Pilot Randomised Control Trial // Acupunct. Med. 2007. Vol. 25, № 1–2. P. 11–17.
Tong Y., Guo H., Han B. Fifteen-day Acupuncture Treatment Relieves Diabetic Peripheral Neuropathy // J. Acupunct. Meridian Stud. 2010. Vol. 3, № 2. P. 95–103.
Yeh B.-Y. et al. Acupuncture helps to regain the consciousness of a COVID-19 patient complicated with hypoxic-ischemic encephalopathy: a case report. // Neurol. Sci. 2021. Vol. 42, № 2. P. 475–478.
Su H.-H., Cui H.-S., Su H.-L. [Effect of Acupuncture at Thirteen Evil Acupoints on Liver Function, and the Contents of Blood Ammonia and β-endorphin in Patients with Hepatic Encephalopathy]. // Zhen ci yan jiu = Acupunct. Res. 2017. Vol. 42, № 4. P. 342–345.
Yu B. et al. Acupuncture treatment of diabetic peripheral neuropathy: An overview of systematic reviews // J. Clin. Pharm. Ther. 2021. Vol. 46, № 3. P. 585–598.
Tseng P.-T. et al. The comparative evidence of efficacy of non-invasive brain and nerve stimulation in diabetic neuropathy: a systematic review and network meta-analysis // J. Neuroeng. Rehabil. 2025. Vol. 22, № 1. P. 88.
Jin D. et al. Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: A meta-analysis of randomized controlled trials // Diabetes Res. Clin. Pract. 2010. Vol. 89, № 1. P. 10–15.
Sánchez-Rodríguez E.C., López V.J. Hypoxic ischemic encephalopathy (HIE). // Front. Neurol. 2024. Vol. 15. P. 1389703.
Weng J. et al. Efficacy and safety of hyperbaric oxygen therapy for diabetes peripheral neuropathy: A systematic review and meta-analysis. // Medicine (Baltimore). 2024. Vol. 103, № 36. P. e39699.
Jiang F. et al. Quality of evidence supporting the role of hyperbaric oxygen therapy for diabetic foot ulcers. // Int. Wound J. 2024. Vol. 21, № 4. P. e14530.
Health Quality Ontario. Hyperbaric Oxygen Therapy for the Treatment of Diabetic Foot Ulcers: A Health Technology Assessment. // Ont. Health Technol. Assess. Ser. 2017. Vol. 17, № 5. P. 1–142.
Sharma R. et al. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcer, a systematic review and meta-analysis of controlled clinical trials // Sci. Rep. 2021. Vol. 11, № 1. P. 2189.
MOREIRA DA CRUZ D.L., OLIVEIRA-PINTO J., MANSILHA A. The role of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers: a systematic review with meta-analysis of randomized controlled trials on limb amputation and ulcer healing // Int. Angiol. 2022. Vol. 41, № 1.
Elraiyah T. et al. A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. // J. Vasc. Surg. 2016. Vol. 63, № 2 Suppl. P. 46S-58S.e1-2.
Brouwer R.J. et al. A systematic review and meta-analysis of hyperbaric oxygen therapy for diabetic foot ulcers with arterial insufficiency. // J. Vasc. Surg. 2020. Vol. 71, № 2. P. 682-692.e1.
Oyebode O.A., Jere S.W., Houreld N.N. Current Therapeutic Modalities for the Management of Chronic Diabetic Wounds of the Foot. // J. Diabetes Res. 2023. Vol. 2023. P. 1359537.
Huang E.T. et al. A clinical practice guideline for the use of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers. // Undersea Hyperb. Med. 2015. Vol. 42, № 3. P. 205–247.
Everett E., Mathioudakis N. Update on management of diabetic foot ulcers. // Ann. N. Y. Acad. Sci. 2018. Vol. 1411, № 1. P. 153–165.
Damineni U. et al. Clinical Outcomes of Hyperbaric Oxygen Therapy for Diabetic Foot Ulcers: A Systematic Review. // Cureus. 2025. Vol. 17, № 2. P. e78655.
Canha F., Soares R. The use of innovative targeted angiogenic therapies for ischemic diabetic foot ulcer repair: From nanomedicine and microRNAs toward hyperbaric oxygen therapy. // Porto Biomed. J. 2023. Vol. 8, № 1. P. e187.
Zhang Z. et al. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcers: An updated systematic review and meta-analysis // Asian J. Surg. 2022. Vol. 45, № 1. P. 68–78.
Higgins D.M. et al. A randomized controlled trial of cognitive behavioral therapy compared with diabetes education for diabetic peripheral neuropathic pain // J. Health Psychol. 2022. Vol. 27, № 3. P. 649–662.
Психиатрия и наркология: учеб.-метод. пособие / ed. Шилова О.В. Х.С.О.. Х.Н.В. Гомель: ГомГМУ, 2015. Vol. 88.
Всемирная организация здравоохранения. Тест RUS-AUDIT для выявления расстройств, обусловленных употреблением алкоголя [Electronic resource] // https://gkbyudina.ru/storage/uploads/docs/RUS-AUDIT.pdf?ysclid=lw1qhanswg599205969 . 2021.
Berman A.H., Bergman H., Palmstierna T. DUDIT (The Drug Use Disorders Identification Test) Manual Version 1.0. Stockholm: Karolinska Institutet, Department of Clinical Neuroscience; 2003. 12 p. [Electronic resource].
Wijdicks E.F.M. et al. Validation of a new coma scale: The FOUR score // Ann. Neurol. 2005. Vol. 58, № 4. P. 585–593.
Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. // J. Psychiatr. Res. 1975. Vol. 12, № 3. P. 189–198.
Khramilin V.N. et al. Diagnosis of diabetic polyneuropathy in primary care // Russ. J. Pain. 2021. Vol. 19, № 2. P. 47.
Мисникова И.В. Ведение пациентов с сахарным диабетом и сопутствующими осложнениями: диабетической нейропатией и цереброваскулярной болезнью // Эффективная фармакотерапия. 2018. Vol. 4, № 30. P. 24–33.
Yoshimura M., Furue H. In vivo electrophysiological analysis of mechanisms of monoaminergic pain inhibitory systems // Pain. 2017. Vol. 158, № 1. P. S85–S91.
Neufeld M. et al. Validation of a screening test for alcohol use, the Russian Federation // Bull. World Health Organ. 2021. Vol. 99, № 7. P. 496–505.
Dutra L.M.A. et al. Is it possible to substitute the monofilament test for the Ipswich Touch Test in screening for peripheral diabetic neuropathy? // Diabetol. Metab. Syndr. 2020. Vol. 12, № 1. P. 27.
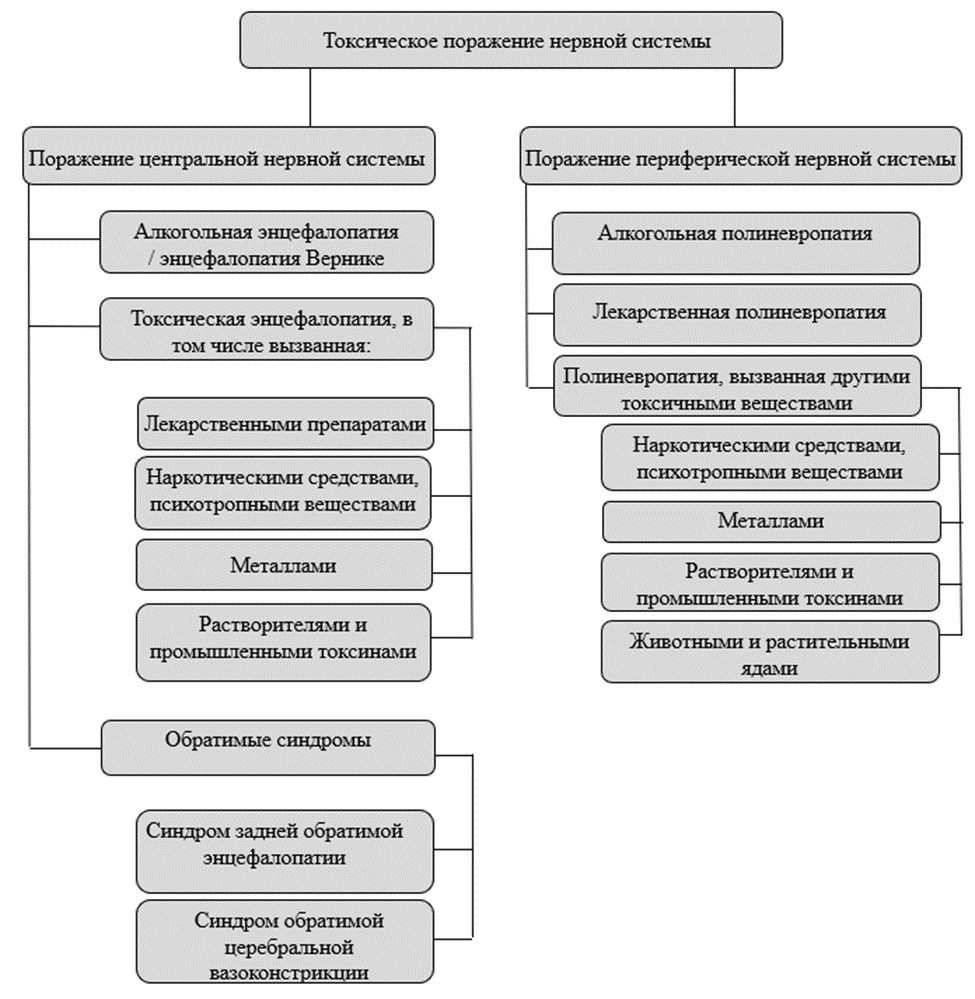 Рисунок 1. Классификация токсических поражений нервной системы
Рисунок 1. Классификация токсических поражений нервной системы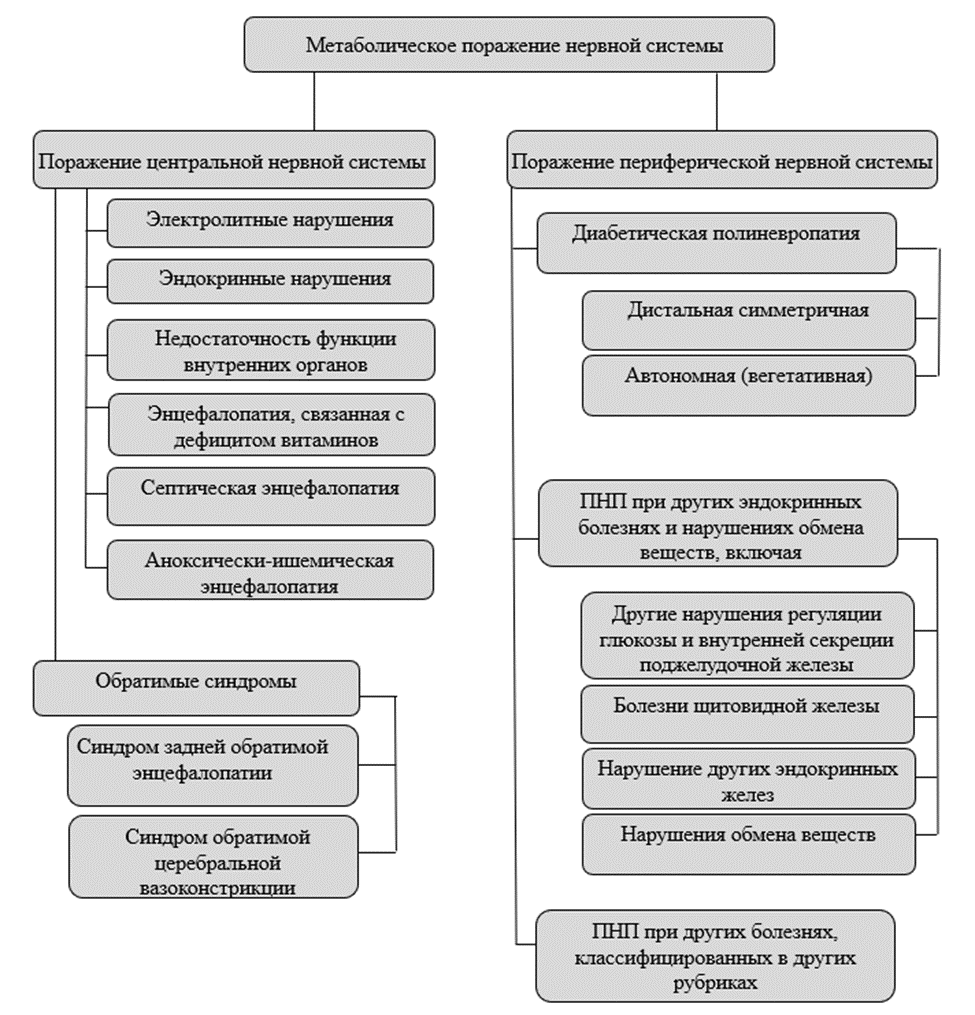 Рисунок 2. Классификация метаболических поражений нервной системы
Рисунок 2. Классификация метаболических поражений нервной системы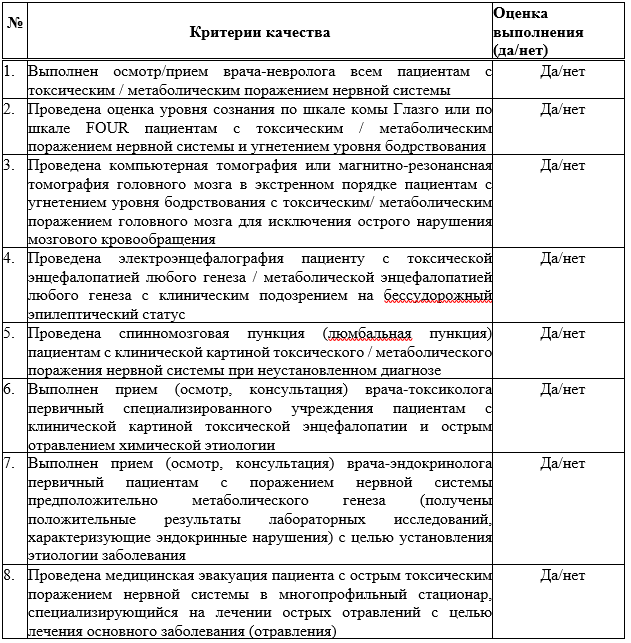
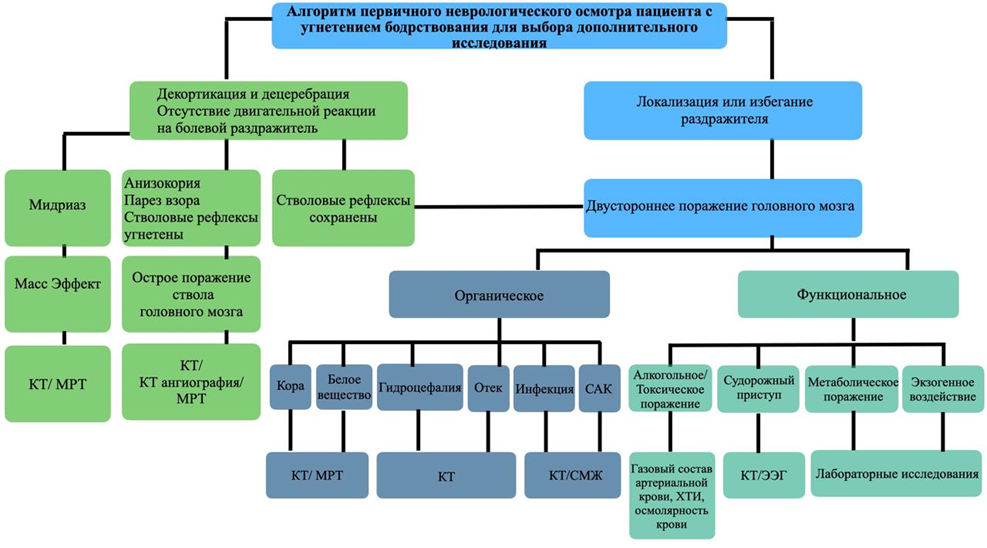 Приложение Б2 Диагностический алгоритм действий врача при подозрении на токсико-метаболическую энцефалопатию [8]
Приложение Б2 Диагностический алгоритм действий врача при подозрении на токсико-метаболическую энцефалопатию [8]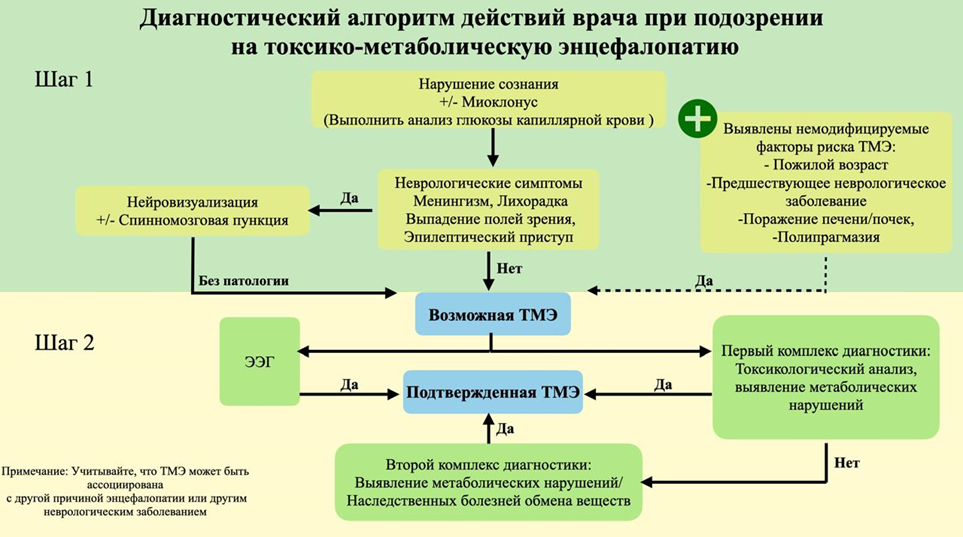 Приложение Б3 Алгоритм оказания медицинской помощи пациенту с энцефалопатией Вернике [2]:
Приложение Б3 Алгоритм оказания медицинской помощи пациенту с энцефалопатией Вернике [2]: