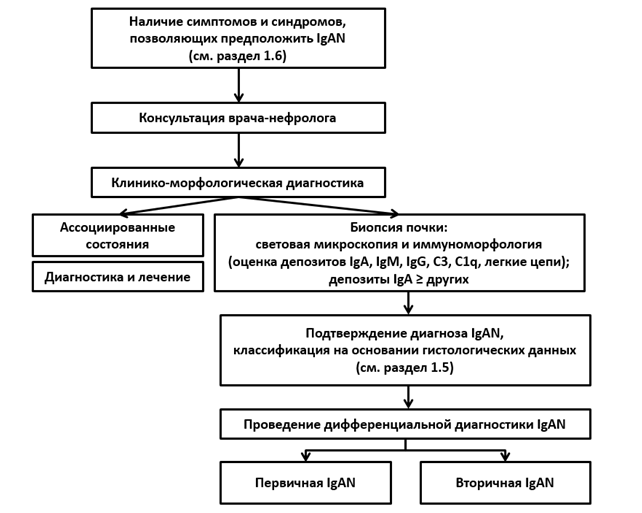
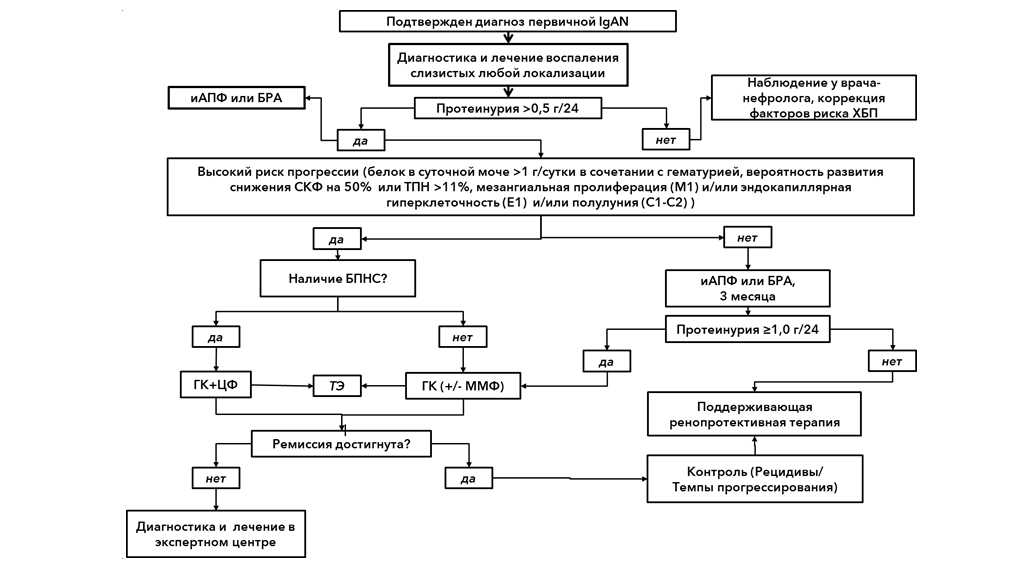
1. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2012;2:139-274. https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf
2.Нефрология. Клинические рекомендации. По ред. Шилов ЕМ, Смирнов АВ, Козловская НЛ. ГОЭТАР-Медиа, 2016
3. Schena FP, Nistor I. Epidemiology of IgA Nephropathy: A Global Perspective. Semin Nephrol 2018;38:435-442. doi: 10.1016/j.semnephrol.2018.05.013
4. Reily C, Ueda H, Huang ZQ et al. Cellular Signaling and Production of Galactose-Deficient IgA1 in IgA Nephropathy, an Autoimmune Disease. Journal of Immunology Research 2014(2):197548. doi: 10.1155/2014/197548
5. Hiki Y, Odani H, Takahashi M et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 2001;59(3):1077-1085. doi: 10.1046/j.1523-1755.2001.0590031077.x
6.Tomana M, Novak J, Julian B et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 1999;104(1):73-81. doi: 10.1172/JCI5535
7. Boyaka PN. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J Immunol 2017;199:9-16. doi: 10.4049/jimmunol.1601775
8.Muto M, Manfroi B, Suzuki H et al. Toll-Like Receptor 9 Stimulation Induces Aberrant Expression of a Proliferation-Inducing Ligand by Tonsillar Germinal Center B Cells in IgA Nephropathy. J Am Soc Nephrol 2017;28(4):1227-1238. doi: 10.1681/ASN.2016050496
9. Robert T, Berthelot L, Cambier A et al. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trend Mol Med 2015;12:762-775. doi: 10.1016/j.molmed.2015.10.003
10. Ben Mkaddem S, Benhamou M, Monteiro RC. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front Immunol 2019;10:1-12. doi: 10.3389/fimmu.2019.00811
11. Novak J, Julian BA, Tomana M et al. IgA Glycosylation and IgA Immune Complexes in the Pathogenesis of IgA Nephropathy. Semin Nephrol 2008;28(1):78-87. doi: 10.1016/j.semnephrol.2007.10.009
12. Wyatt RJ, Julian BA. IgA Nephropathy. N Engl J Med 2013;368:2402-2414. doi: 10.1056/NEJMra1206793
13. Novak J, Tomana M, Matousovic K et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 2005;67:504-513. doi: 10.1111/j.1523-1755.2005.67107.x
14. Kiryluk K, Li Y, Sanna-Cherchi S et al. Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis. PLoS Genet 2012;8(6):e1002765. doi: 10.1371/journal.pgen.1002765
15. Moriyama T, Tanaka K, Iwasaki C et al. Prognosis in IgA Nephropathy: 30-Year Analysis of 1,012 Patients at a Single Center in Japan. PLOS ONE 2014;9(3):e91756. doi: 10.1371/journal.pone.0091756
16. Lee H, Kim DK, Oh KH et al. Mortality of IgA Nephropathy Patients: A Single Center in Korea. Experience over 30 Years. PLoS ONE 2012;7(12):e51225. doi: 10.1371/journal.pone.0051225
17. Le W, Liang S, Hu Y et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 2012;27:1479-1485. doi: 10.1093/ndt/gfr527
18. Coppo R, Troyanov S, Bellur S et al. Validation of the Oxford classification of IgA-nephropathy in cohorts with different presentations and treatments. Kidney Int 2014;86:828-836. doi: 10.1038/ki.2014.63
19. Добронравов ВА, Мужецкая ТО, Лин ДИ, Кочоян ЗШ. Иммуноглобулин А-нефропатия в российской популяции: клинико-морфологическая презентация и отдаленный прогноз. Нефрология 2019;23(6):45-60. doi: 10.36485/1561-6274-2019-236-45-60
20. Сурсякова КИ, Сафьянова ТВ. Некоторые эпидемиологические особенности заболеваемости гломерулярными и тубулоинтерстициальными болезнями почек и инфекциями мочевыводящих путей в Алтайском крае. Экспериментальная и клиническая урология 2017;4:6-11
21. Yeo SC, Goh SM, Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology (Carlton) 2019;24(9):885-895. doi: 10.1111/nep.13592
22. Chang JH, Kim DK, Kim HW et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant 2009;24(8):2406-2410. doi: 10.1093/ndt/gfp091
23. Андрусев АМ, Томилина НА, Перегудова НГ, Шинкарев МБ. Заместительная терапия терминальной хронической почечной недостаточности в Российской Федерации 2014-2018 гг. Отчет по данным Общероссийского Регистра заместительной почечной терапии Российского диализного общества. http://nephro.ru/index.php?r=site/pageView&id=298%20,%20journal.nephro.ru/index.php?r=journal/pageView&id=298
24. Хроническая болезнь почек (ХБП). Клинические рекомендации. https://rusnephrology.org/wp-content/uploads/2020/12/CKD_final.pdf
25. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604-12. doi: 10.7326/0003-4819-150-9-200905050-00006
26. Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010;55(4):622-7. doi: 10.1053/j.ajkd.2010.02.337
27. Cattran DC, Coppo R, Cook HT et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. A Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Kidney Int 2009;76(5): 534-545. doi: 10.1038/ki.2014.63
28. Trimarchi H, Barratt J, Cattran DC et al. IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017;91(5):1014-1021. doi: 10.1016/j.kint.2017.02.003
29. Barbour SJ, Coppo R, Zhang H et al. Evaluating a New International Risk-Prediction Tool in IgA Nephropathy. JAMA Intern Med 2019;179(7):942-952. doi: 10.1001/jamainternmed.2019.0600
30. Wang M, Lv J, Zhang X et al. Secondary IgA Nephropathy Shares the Same Immune Features With Primary IgA Nephropathy. Kidney Int Rep 2019;5(2):165-172. doi: 10.1016/j.ekir.2019.10.012
31. Saha MK, Julian BA, Novak J, Rizk DV. Secondary IgA nephropathy. Kidney Int 2018;94(4):674-681. doi: 10.1016/j.kint.2018.02.030
32. Liu LL, Wang LN, Jiang Y et al. Tonsillectomy for IgA nephropathy: a meta-analysis. Am J Kidney Dis 2015;65(1):80-7. doi: 10.1053/j.ajkd.2014.06.036
33. Duan J, Liu D, Duan G, Liu Z. Long-term efficacy of tonsillectomy as a treatment in patients with IgA nephropathy: a meta-analysis. Int Urol Nephrol 2017;49(1):103-112. doi: 10.1007/s11255-016-1432-7
34. Inker LA, Mondal H, Greene T et al. Early Change in Urine Protein as a Surrogate End Point in Studies of IgA Nephropathy: An Individual-Patient Meta-analysis. Am J Kidney Dis 2016;68(3):392-401. doi: 10.1053/j.ajkd.2016.02.042
35. Thompson A, Carroll K, A Inker L et al. Proteinuria Reduction as a Surrogate End Point in Trials of IgA Nephropathy. Clin J Am Soc Nephrol 2019;14(3):469-481. doi: 10.2215/CJN.08600718
36. He P, Wang H, Huang C, He L. Hematuria was a high risk for renal progression and ESRD in immunoglobulin a nephropathy: a systematic review and meta-analysis. Ren Fail 2021;43(1):488-499. doi: 10.1080/0886022X.2021.1879852
37. Offringa M, Benbassat J. The value of urinary red cell shape in the diagnosis of glomerular and post-glomerular haematuria. A meta-analysis. Postgrad Med J 1992;68(802):648-54. doi: 10.1136/pgmj.68.802.648
38. Bi TD, Zheng JN, Zhang JX et al. Serum complement C4 is an important prognostic factor for IgA nephropathy: a retrospective study. BMC Nephrol 2019;20(1):244. doi: 10.1186/s12882-019-1420-0
39. Pan M, Zhou Q, Zheng S et al. Serum C3/C4 ratio is a novel predictor of renal prognosis in patients with IgA nephropathy: a retrospective study. Immunol Res 2018;66(3):381-391. doi: 10.1007/s12026-018-8995-6
40. Pan M, Zhang J, Li Z et al. Increased C4 and decreased C3 levels are associated with a poor prognosis in patients with immunoglobulin A nephropathy: a retrospective study. BMC Nephrol 2017;18(1):231. doi: 10.1186/s12882-017-0658-7
41. Zhang Y, Duan SW, Chen P et al. Relationship between serum C3/C4 ratio and prognosis of immunoglobulin A nephropathy based on propensity score matching. Chin Med J (Engl) 2020;133(6):631-637. doi: 10.1097/CM9.0000000000000674
42. Zhang J, Wang C, Tang Y et al. Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton) 2013;18(2):125-31. doi: 10.1111/nep.12010
43. Lv J, Shi S, Xu D et al. Evaluation of the Oxford Classification of IgA nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 2013;62(5):891-9. doi: 10.1053/j.ajkd.2013.04.021
44. Shao X, Li B, Cao L et al. Evaluation of crescent formation as a predictive marker in immunoglobulin A nephropathy: a systematic review and meta-analysis. Oncotarget 2017;8(28):46436-46448. doi: 10.18632/oncotarget.17502
45. Luo MN, Yao CW, Xu BH et al. Continuation of immunosuppressive treatment may be necessary in IgA nephropathy patients with remission of proteinuria: Evaluation by repeat renal biopsy. Exp Ther Med 2014;7(3):553-559. doi: 10.3892/etm.2013.1467
46. Shen XH, Liang SS, Chen HM et al. Reversal of active glomerular lesions after immunosuppressive therapy in patients with IgA nephropathy: a repeat-biopsy based observation. J Nephrol 2015;28(4):441-9. doi: 10.1007/s40620-014-0165-x
47. Luo MN, Pan Q, Huang ZQ et al. IgA nephropathy patients with partial crescent formation benefit from immunosuppressants: evidence from repeat renal biopsy. Discov Med 2020;30(159):19-25
48. Jullien P, Laurent B, Berthoux F et al. Repeat renal biopsy improves the Oxford classification-based prediction of immunoglobulin A nephropathy outcome. Nephrol Dial Transplant 2020;35(7):1179-1186. doi: 10.1093/ndt/gfy341
49. Khairwa A. Indian scenario of IgA nephropathy: a systematic review and meta-analysis. Afr Health Sci 2021;21(1):159-165. doi: 10.4314/ahs.v21i1.21
50. Okpechi IG, Ameh OI, Bello AK et al. Epidemiology of Histologically Proven Glomerulonephritis in Africa: A Systematic Review and Meta-Analysis. PLoS One 2016;11(3):e0152203. doi: 10.1371/journal.pone.0152203
51. Katafuchi R, Nagae H, Masutani K et al. Comprehensive evaluation of the significance of immunofluorescent findings on clinicopathological features in IgA nephropathy. Clin Exp Nephrol 2019;23(2):169-181. doi: 10.1007/s10157-018-1619-6
52. Tan L, Tang Y, Pei G et al. A multicenter, prospective, observational study to determine association of mesangial C1q deposition with renal outcomes in IgA nephropathy. Sci Rep 2021;11(1):5467. doi: 10.1038/s41598-021-84715-7
53. Wu J, Hu Z, Wang Y et al. Severe glomerular C3 deposition indicates severe renal lesions and a poor prognosis in patients with immunoglobulin A nephropathy. Histopathology 2021;78(6):882-895. doi: 10.1111/his.14318
54. Sun S, Di W, Li R et al. The clinicopathological characteristics and outcomes of IgA nephropathy with predominant lambda or kappa light-chain deposition. Int Urol Nephrol 2021 Nov 18. doi: 10.1007/s11255-021-03062-8
55. Nakamoto Y, Asano Y, Dohi K et al. Primary IgA glomerulonephritis and Schönlein-Henoch purpura nephritis: Clinicopathological and immunohistological characteristics. Q J Med 1978;47(188):495-516
56. Yang P, Zou H, Xiao B, Xu G. Comparative Efficacy and Safety of Therapies in IgA Nephropathy: A Network Meta-analysis of Randomized Controlled Trials. Kidney Int Rep 2018;3(4):794-803. doi: 10.1016/j.ekir.2018.03.006
57. Zhao Y, Fan H, Bao BY. Efficacy and Safety of Renin-Angiotensin Aldosterone System Inhibitor in Patients with IgA Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Iran J Public Health 2019;48(9):1577-1588
58. Ji Y, Yang K, Xiao B, Lin J et al. Efficacy and safety of angiotensin-converting enzyme inhibitors/angiotensin receptor blocker therapy for IgA nephropathy: A meta-analysis of randomized controlled trials. J Cell Biochem 2019;120(3):3689-3695. doi: 10.1002/jcb.27648
59. Han S, Yao T, Lu Y et al. Efficacy and Safety of Immunosuppressive Monotherapy Agents for IgA Nephropathy: A Network Meta-Analysis. Front Pharmacol 2021;11:539545. doi: 10.3389/fphar.2020.539545
60. Liu LJ, Yang YZ, Shi SF et al. Effects of Hydroxychloroquine on Proteinuria in IgA Nephropathy: A Randomized Controlled Trial. Am J Kidney Dis 2019;74(1):15-22. doi: 10.1053/j.ajkd.2019.01.026
61. Eljaaly K, Alireza KH, Alshehri S, Al-Tawfiq JA. Hydroxychloroquine safety: A meta-analysis of randomized controlled trials. Travel Med Infect Dis 2020;36:101812. doi: 10.1016/j.tmaid.2020.101812
62. Rollino C, Vischini G, Coppo R. IgA nephropathy and infections. J Nephrol 2016;29(4):463-8. doi: 10.1007/s40620-016-0265-x
63. Rehnberg J, Symreng A, Ludvigsson JF, Emilsson L. Inflammatory Bowel Disease Is More Common in Patients with IgA Nephropathy and Predicts Progression of ESKD: A Swedish Population-Based Cohort Study. J Am Soc Nephrol 2021;32(2):411-423. doi: 10.1681/ASN.2020060848
64. Liu XZ, Zhang YM, Jia NY, Zhang H. Helicobacter pylori infection is associated with elevated galactose-deficient IgA1 in IgA nephropathy. Ren Fail 2020;42(1):539-546. doi: 10.1080/0886022X.2020.1772295
65. Pipili C, Michopoulos S, Sotiropoulou M et al. Is there any association between IgA nephropathy, Crohn's disease and Helicobacter pylori infection? Ren Fail 2012;34(4):506-9. doi: 10.3109/0886022X.2011.653774
66. Jiang J, Wang XX, Shen PC et al. Clinical investigation of mucosal immune system in IgA nephropathy patients. J Dalian Med Univ 2016;38:558-61
67. Chairatana P, Nolan EM. Defensins, lectins, mucins, and secretory immunoglobulin a: microbe-binding biomolecules that contribute to mucosal immunity in the human gut. Crit Rev Biochem Mol Biol 2017;52:45-56. doi: 10.1080/10409238.2016.1243654
68. Russell MW, Mestecky J, Julian BA, Galla JH. IgA-associated renal diseases: antibodies to environmental antigens in sera and deposition of immunoglobulins and antigens in glomeruli. J Clin Immunol 1986;6:74-86. doi: 10.1007/BF00915367
69. Chang S, Li XK. The Role of Immune Modulation in Pathogenesis of IgA Nephropathy. Front Med (Lausanne) 2020;7:92. doi: 10.3389/fmed.2020.00092
70. Han S, Yao T, Lu Y et al. Efficacy and Safety of Immunosuppressive Monotherapy Agents for IgA Nephropathy: A Network Meta-Analysis. Front Pharmacol 2021;11:539545. doi: 10.3389/fphar.2020.539545
71. Natale P, Palmer SC, Ruospo M et al. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev 2020;3(3):CD003965. doi: 10.1002/14651858
72. Yang P, Wang Q, Xie C et al. Efficacy and Safety of Agents in IgA Nephropathy: An Update Network Meta-Analysis. Kidney Blood Press Res 2018;43(6):1890-1897. doi: 10.1159/000496000
73. Tan J, Dong L, Ye D et al. The efficacy and safety of immunosuppressive therapies in the treatment of IgA nephropathy: A network meta-analysis. Sci Rep 2020;10(1):6062. doi: 10.1038/s41598-020-63170-w
74. Zhang Z, Yang Y, Jiang SM, Li WG. Efficacy and safety of immunosuppressive treatment in IgA nephropathy: a meta-analysis of randomized controlled trials. BMC Nephrol 2019;20(1):333. doi: 10.1186/s12882-019-1519-3
75. Tian L, Shao X, Xie Y et al. The long-term efficacy and safety of immunosuppressive therapy on the progression of IgA nephropathy: a meta-analysis of controlled clinical trials with more than 5-year follow-up. Expert Opin Pharmacother 2015;16(8):1137-47. doi: 10.1517/14656566.2015.1038238
76. Avino LJ, Naylor SM, Roecker AM. Pneumocystis jirovecii Pneumonia in the Non-HIV-Infected Population. Ann Pharmacother 2016;50(8):673-9. doi: 10.1177/1060028016650107
77. Мельниченко ГА, Белая ЖЕ, Рожинская ЛЯ и др. Федеральные клинические рекомендации по диагностике, лечению и профилактике остеопороза. Проблемы эндокринологии 2017;63(6):392-426. doi: 10.14341/probl2017636392-426
78. Добронравов ВА, Кочоян ЗШ, Мужецкая ТО, Лин ДИ. Анализ эффективности терапии иммуноглобулин А-нефропатии. Терапевтический архив 2020;92(6):23-32. doi: 10.26442/00403660.2020.06.000669
79. Кочоян ЗШ, Лиева АЗ, Гальковская ТО, Добронравов ВА. Иммуносупрессия, тонзиллэктомия и ремиссии иммуноглобулин А-нефропатии высокого риска. Терапевтический архив 2024;96(6):600-605. doi: 10.26442/00403660.2024.06.202728
80. Zheng JN, Bi TD, Zhu LB, Liu LL. Efficacy and safety of mycophenolate mofetil for IgA nephropathy: An updated meta-analysis of randomized controlled trials. Exp Ther Med 2018;16(3):1882-1890. doi: 10.3892/etm.2018.6418
81. Liu Y, Xiao J, Shi X et al. Immunosuppressive agents versus steroids in the treatment of IgA nephropathy-induced proteinuria: A meta-analysis. Exp Ther Med 2016;11(1):49-56. doi: 10.3892/etm.2015.2860. Erratum in: Exp Ther Med 2019;17(4):2877
82. Zheng J, Gong X, Wu Z. Immunosuppressive agents in the treatment of IgA nephropathy: A meta-analysis of clinical randomized controlled literature. Niger J Clin Pract 2020;23(4):437-449. doi: 10.4103/njcp.njcp_112_18
83. Ma F, Yang X, Zhou M et al. Treatment for IgA nephropathy with stage 3 or 4 chronic kidney disease: low-dose corticosteroids combined with oral cyclophosphamide. J Nephrol 2020;33(6):1241-1250. doi: 10.1007/s40620-020-00752-x
84. Du B, Jia Y, Zhou W, Min X, Miao L, Cui W. Efficacy and safety of mycophenolate mofetil in patients with IgA nephropathy: an update meta-analysis. BMC Nephrol 2017;18(1):245. doi: 10.1186/s12882-017-0647-x
85. Chen Y, Li Y, Yang S, Li Y, Liang M. Efficacy and safety of mycophenolate mofetil treatment in IgA nephropathy: a systematic review. BMC Nephrol 2014;15:193. doi: 10.1186/1471-2369-15-193
86. Schena FP, Montenegro M, Scivittaro V. Meta-analysis of randomised controlled trials in patients with primary IgA nephropathy (Berger's disease). Nephrol Dial Transplant 1990;5 Suppl 1:47-52. doi: 10.1093/ndt/5.suppl_1.47
87. Sarcina C, Tinelli C, Ferrario F et al. Changes in Proteinuria and Side Effects of Corticosteroids Alone or in Combination with Azathioprine at Different Stages of IgA Nephropathy. Clin J Am Soc Nephrol 2016;11(6):973-81. doi: 10.2215/CJN.02300215
88. Liu T, Wang Y, Mao H et al. Efficacy and safety of immunosuppressive therapies in the treatment of high-risk IgA nephropathy: A network meta-analysis. Medicine (Baltimore) 2021;100(8):e24541. doi: 10.1097/MD.0000000000024541
89. Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 2002;13(1):142-8
90. McIntyre CW, Fluck RJ, Lambie SH. Steroid and cyclophosphamide therapy for IgA nephropathy associated with crescenteric change: an effective treatment. Clin Nephrol 2001;56(3):193-8
91. Tumlin JA, Lohavichan V, Hennigar R. Crescentic, proliferative IgA nephropathy: clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant 2003;18(7):1321-1329
92. Ramachandran R, Doddi P, Nandakrishna B et al. Combination of pulse cyclophosphamide and steroids in crescentic IgA nephropathy. Int Urol Nephrol 2015;47(11):1917-1918. doi: 10.1007/s11255-015-1076-z
93. Liu X, Dewei D, Sun S et al. Treatment of severe IgA nephropathy: mycophenolate mofetil/prednisone compared to cyclophosphamide/prednisone. Int J Clin Pharmacol Ther 2014;52(2):95-102. doi: 10.5414/CP201887
94. Hernández-Rodríguez J, Carbonell C, Mirón-Canelo JA et al. Rituximab treatment for IgA vasculitis: A systematic review. Autoimmun Rev 2020;19(4):102490. doi: 10.1016/j.autrev.2020.102490
95. Острое повреждение почек (ОПП). Клинические рекомендации. https://rusnephrology.org/wp-content/uploads/2020/12/AKI_final.pdf
96. Liu X, Dewei D, Sun S et al. Treatment of severe IgA nephropathy: mycophenolate mofetil/prednisone compared to cyclophosphamide/prednisone. Int J Clin Pharmacol Ther 2014;52(2):95-102. doi: 10.5414/CP201887
97. Liu XW, Li DM, Xu GS, Sun SR. Comparison of the therapeutic effects of leflunomide and mycophenolate mofetil in the treatment of immunoglobulin a nephropathy manifesting with nephrotic syndrome. Int J Clin Pharmacol Ther 2010;48(8):509-513. doi: 10.5414/CPP48509
98. Chen X, Chen P, Cai G et al. A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy. Int J Clin Pharmacol Ther 2014;52(2):95-102. doi: 10.5414/CP201887
99. Hou J-H, Le W-B, Chen N et al. Mycophenolate Mofetil Combined With Prednisone Versus Full-Dose Prednisone in IgA Nephropathy With Active Proliferative Lesions: A Randomized Controlled Trial. Am J Kidney Dis 2017;69(6):788-795. doi: 10.1053/j.ajkd.2016.11.027
100. Lau KK, Wyatt RJ, Moldoveanu Z et al. Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol 2007;22:2067
101. Kiryluk K, Sanchez-Rodriguez E, Zhou XJ et al. Genome-wide association analyses define pathogenic signaling pathways and prioritize drug targets for IgA nephropathy. Nat Genet 2023;55(7):1091-1105. doi: 10.1038/s41588-023-01422-x
102. Willey CJ, Coppo R, Schaefer F et al. The incidence and prevalence of IgA nephropathy in Europe. Nephrol Dial Transplant 2023;38(10):2340-2349. doi: 10.1093/ndt/gfad082
103. Utsunomiya Y, Koda T, Kado T et al. Incidence of pediatric IgA nephropathy. Pediatr Nephrol 2003;18(6):511-5. doi: 10.1007/s00467-003-1127-z
104. Хроническая болезнь почек. Клинические рекомендации, 2022 год, возрастная категория: дети. https://cr.minzdrav.gov.ru/recomend/713_1
105. Selewski DT, Ambruzs JM, Appel GB et al. Clinical Characteristics and Treatment Patterns of Children and Adults With IgA Nephropathy or IgA Vasculitis: Findings From the CureGN Study. Kidney Int Rep 2018;3:1373
106. Yata N, Nakanishi K, Shima Y et al. Improved renal survival in Japanese children with IgA nephropathy. Pediatr Nephrol 2008;23:905
107. Su B, Jiang Y, Li Z et al. Are children with IgA nephropathy different from adult patients? Pediatr Nephrol 2024;39(8):2403-2412. doi: 10.1007/s00467-024-06361-1
108. Barbour SJ, Coppo R, Er L et al. Updating the International IgA Nephropathy. Prediction Tool for use in children. Kidney Int 2021;99:1439
109. https://www.uptodate.com/contents/iga-nephropathy-clinical-features-and-diagnosis. IgA nephropathy: Clinical features and diagnosis
110. Maixnerová D, Tesař V, Ryšavá R et al. The coincidence of IgA nephropathy and Fabry disease. BMC Nephrol 2013;14:6. doi: 10.1186/1471-2369-14-6
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 2021;100(4S):S1-S276. doi: 10.1016/j.kint.2021.05.021
112. Vivarelli M, Samuel S, Coppo R et al.; International Pediatric Nephrology Association. IPNA clinical practice recommendations for the diagnosis and management of children with IgA nephropathy and IgA vasculitis nephritis. Pediatr Nephrol 2024. doi: 10.1007/s00467-024-06502-6
113. Shima Y, Nakanishi K, Hama T et al. Spontaneous remission in children with IgA nephropathy. Pediatr Nephrol 2013;28:71-76. doi: 10.1007/s00467-012-2294-6
114. Yang Y, Ohta K, Shimizu M et al. Treatment with low-dose angiotensin-converting enzyme inhibitor (ACEI) plus angiotensin II receptor blocker (ARB) in pediatric patients with IgA nephropathy. Clin Nephrol 2005;64:35-40. doi: 10.5414/cnp64035
115. Ellis D, Vats A, Moritz ML, et al. Long-term antiproteinuric and renoprotective efficacy and safety of losartan in children with proteinuria. J Pediatr 2003;143(1):89-97. doi: 10.1016/S0022-3476(03)00279-8
116. Hyun H, Ahn YH, Park E, et al. Efficacy and safety of losartan in childhood immunoglobulin A nephropathy: a prospective multicenter study. Child Kidney Dis 2023;27(2):97-104. doi: 10.3339/ckd.23.006
117. Nakanishi K, Iijima K, Ishikura K et al. Efficacy and safety of lisinopril for mild childhood IgA nephropathy: a pilot study. Pediatr Nephrol 2009;24:845-849. doi: 10.1007/s00467-008-1006-8
118. Shima Y, Nakanishi K, Sako M, et al. Lisinopril versus lisinopril and losartan for mild childhood IgA nephropathy: a randomized controlled trial (JSKDC01 study). Pediatr Nephrol 2019;34(5):837-846. doi: 10.1007/s00467-018-4099-8
119. Enalapril: Drug information 2024 UpToDate https://medilib.ir/uptodate/show/9405
120. Trautmann A, Boyer O, Hodson E et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 2023;38:877-919. doi: 10.1007/s00467-022-05739-3
121. Trautmann A, Vivarelli M, Samuel S et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 2020;35(8):1529-1561. doi: 10.1007/s00467-020-04519-1
122. Yoshikawa N, Honda M, Iijima K et al. Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol 2006;1:511-517
123. Wong MG, Lv J, Hladunewich MA, et al. The Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING) Study: Trial Design and Baseline Characteristics. Am J Nephrol 2021;52(10-11):827-836. doi: 10.1159/000519812
124. Lv J, Wong MG, Hladunewich MA, et al. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2022;327(19):1888-1898. doi: 10.1001/jama.2022.5368
125. Venettacci O, Larkins N, Willis F. Childhood IgA Nephropathy Successfully Treated with Targeted-Release Budesonide: A Case Report. J Paediatr Child Health 2018;54(12):1403. doi: 10.1111/jpc.14259
126. Antonucci L, Colucci M, Emma F, Vivarelli M. A pediatric case of IgA nephropathy benefitting from targeted release formulation–budesonide. Pediatr Nephrol 2023;38:3849-3852. doi: 10.1007/s00467-023-05968-0
127. Mao Y, Zhou W, Zhou Z, et al. Treatment and outcome of IgA nephropathy in children from one single center experience. BMC Pediatr 2023;23(1):377. doi: 10.1186/s12887-023-04195-8
Cambier A, Rabant M, Peuchmaur M et al. Immunosuppressive treatment in children with IgA nephropathy and the clinical value of podocytopathic features. Kidney Int Rep 2018;3:916-925
129. Roccatello D, Ferro M, Coppo R et al. Report on intensive treatment of extracapillary glomerulonephritis with focus on crescentic IgA nephropathy. Nephrol Dial Transplant 1995;10:2054-2059
130. Jiang X-Y, Mo Y, Sun L-Z et al. Efficacy of Methylprednisolone, Cyclophosphamide in Pediatric IgA Nephropathy Assessed by Renal Biopsy. Clin Nephrol 2009;71:625-631
131. Hogg RJ, Bay RС, Jennette JC et al. Randomized Controlled Trial of Mycophenolate Mofetil in Children, Adolescents, and Adults with IgA Nephropathy. Am J Kidney Dis 2015;66:783-791
132. Kang Z, Li Z, Duan C et al. Mycophenolate mofetil therapy for steroid-resistant IgA nephropathy with the nephrotic syndrome in children. Pediatr Nephrol 2015;30:1121-1129
133. Ананьин ПВ, Милованова ТВ, Вашурина ТВ. Течение IgA-нефропатии у детей и ограниченные возможности иммуносупрессивной терапии. Педиатрия. Журнал им. Г.Н. Сперанского 2023;102(4):40-45
134. Shin JI, Lim BJ, Kim PK et al. Effects of cyclosporin A therapy combined with steroids and angiotensin converting enzyme inhibitors on childhood IgA nephropathy. J Korean Med Sci 2010;25(5):723-7. doi: 10.3346/jkms.2010.25.5.723
135. Song Y-H, Cai G-Y, Xiao Y-F et al. Efficacy and safety of calcineurin inhibitor treatment for IgA nephropathy: A meta-analysis. BMC Nephrol 2017;18:61
136. Zhang J-J, Wang Q, Dou W-J et al. Clinical effect of tacrolimus combined with glucocorticoid in the treatment of IgA nephropathy in children. Zhongguo Dang Dai Er Ke Za Zhi Chin J Contemp Pediatr 2019;21:265-270
137. Zhang Y, Luo J, Hu B et al. Efficacy and safety of tacrolimus combined with glucocorticoid treatment for IgA nephropathy: A meta-analysis. J Int Med Res 2018;46:3236-3250. doi: 10.1177/0300060518776566
138. Kawamura T, Yoshimura M, Miyazaki Y et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant 2014;29(8):1546-53. doi: 10.1093/ndt/gfu020
139. Kawasaki Y, Suyama K, Abe Y et al. Tonsillectomy with methylprednisolone pulse therapy as rescue treatment for steroid-resistant IgA nephropathy in children. Tohoku J Exp Med 2009;218(1):11-6. doi: 10.1620/tjem.218.11
140. Kawasaki Y. Treatment strategy with multidrug therapy and tonsillectomy pulse therapy for childhood-onset severe IgA nephropathy. Clin Exp Nephrol 2022;26(6):501-511. doi: 10.1007/s10157-022-02187-z
141. Enya T, Miyazaki K, Miyazawa T et al. Early tonsillectomy for severe immunoglobulin A nephropathy significantly reduces proteinuria. Pediatr Int 2020;62(9):1054-1057. doi: 10.1111/ped.14264
142. Matsuzaki K, Suzuki H, Kikuchi M et al. Current treatment status of IgA nephropathy in Japan: a questionnaire survey. Clin Exp Nephrol 2023;27(12):1032-1041. doi: 10.1007/s10157-023-02396-0
143. Isbel NM. Glomerulonephritis Management in general practice. Aust Fam Physician 2005;34(11):907-13
144. Методические указания МУ 3.3.1.1095-02 «Медицинские противопоказания к проведению профилактических прививок препаратами национального календаря прививок», утв. 09.01.2002 г.
145. Leow EH, Chong SL, Yap CJY et al. IgA nephropathy in children: before and after the start of COVID-19. Pediatr Nephrol 2024;39(4):1161-1167. doi: 10.1007/s00467-023-06196-2
146. Wu HHL, Shenoy M, Kalra PA, Chinnadurai R. Intrinsic Kidney Pathology in Children and Adolescents Following COVID-19 Vaccination: A Systematic Review. Children (Basel) 2022;9(10):1467. doi: 10.3390/children9101467
147. Ma Y, Xu G. New-onset IgA nephropathy following COVID-19 vaccination. QJM 2023;116(1):26-39. doi: 10.1093/qjmed/hcac185
148. Chen CH, Chiu YW, Chen BD et al. De Novo Biopsy-Proven Glomerular Disease Following COVID-19 Vaccination. J Clin Med 2024;13(15):4494. doi: 10.3390/jcm13154494
149. Sasinka MA, Podracka L, Boor A et al. Enalapril treatment of proteinuria in normotensive children. Bratisl Lek Listy 1999;100(9):476-80
150. Zhang J, Lu X, Feng J et al. Effects of Hydroxychloroquine on Proteinuria in IgA Nephropathy: A Systematic Review and Meta-Analysis. Biomed Res Int 2021 Dec 3;2021:9171715. doi: 10.1155/2021/9171715
151. Liu LJ, Yang YZ, Shi SF et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. American Journal of Kidney Diseases 2019;74(1):15-22. doi: 10.1053/j.ajkd.2019.01.026
152. Tang C, Lv JC, Shi SF et al. Long-term safety and efficacy of hydroxychloroquine in patients with IgA nephropathy: a single-center experience. Journal of Nephrology 2021. doi: 10.1007/s40620-021-00988-1
153. Keskinis Сh, Moysidou E, Christodoulou M et al. Diagnosing and Treating IgAN: Steroids, Budesonide, or Maybe Both? Diagnostics 2024;14(5):512. doi: 10.3390/diagnostics14050512
154. Hotta O, Miyazaki M, Furuta T et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis 2001;38(4):736-43. doi: 10.1053/ajkd.2001.27690
155. Laranjinha I, Matias P, Cassis J et al. IGA nephropathy - Are intravenous steroid pulses more effective than oral steroids in relapse prevention? Nefrologia (Engl Ed) 2018;38(4):355-360. doi: 10.1016/j.nefro.2017.08.004