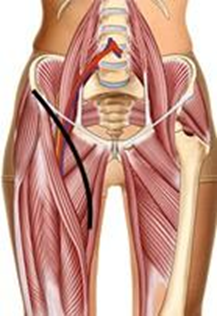
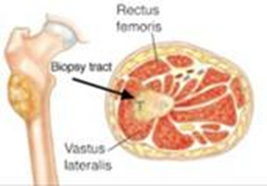
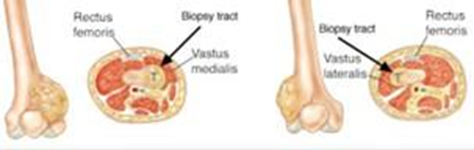
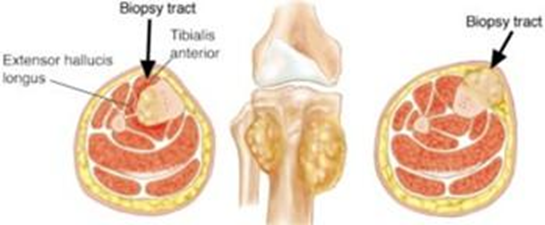
1. WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Soft tissue and bone tumours, 5th ed. Vol. 3. Lyon: IARC; 2020.
2. Stiller CA, Bielack SS, Jundt G et al. Bone tumours in European children and adolescents, 1978–1997. Report form the Automated Childhood Cancer Infromation System project. Eur J Cancer 2006; 42: 2124–2135.
3. Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C, Triche TJ, et al. The Ewing family of tumors--a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994 Aug 4;331(5):294-9. doi: 10.1056/NEJM199408043310503. PMID: 8022439.
4. Calvert GT, Randall RL, Jones KB, Cannon-Albright L, Lessnick S, Schiffman JD. At-risk populations for osteosarcoma: the syndromes and beyond. Sarcoma. 2012;2012:152382. doi: 10.1155/2012/152382. Epub 2012 Mar 12. PMID: 22550413; PMCID: PMC3329649.
5. Kalra S, Grimer RJ, Spooner D, Carter SR, Tillman RM, Abudu A. Radiation-induced sarcomas of bone: factors that affect outcome. J Bone Joint Surg Br. 2007 Jun;89(6):808-13. doi: 10.1302/0301-620X.89B6.18729. PMID: 17613509.
6. SEER Cancer Statistics Fact Sheets: Bone and Joint Cancer. Bethesda, MD: National Cancer Institute; 2021. Available at: http://seer.cancer.gov/statfacts/html/bones.html. Accessed September 8, 2021.
7. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009 Apr 1;115(7):1531-43. doi: 10.1002/cncr.24121. PMID: 19197972; PMCID: PMC2813207.
8. Gurney J.G., Young J.L., Roffers S.D. Malignant Bone Tumors // Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995, National Cancer Institute, SEER Program. 1999. P. 99–110.
9. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Version 2.2024, 03/12/24. National Comprehensive Cancer Network (NCCN)
10. Chow WA. Chondrosarcoma: biology, genetics, and epigenetics. F1000Res. 2018 Nov 20;7:F1000 Faculty Rev-1826. doi: 10.12688/f1000research.15953.1. PMID: 30519452; PMCID: PMC6248264.
11. El Abiad JM, Robbins SM, Cohen B, Levin AS, Valle DL, Morris CD, de Macena Sobreira NL. Natural history of Ollier disease and Maffucci syndrome: Patient survey and review of clinical literature. Am J Med Genet A. 2020 May;182(5):1093-1103. doi: 10.1002/ajmg.a.61530. Epub 2020 Mar 7. PMID: 32144835; PMCID: PMC8164175.
12. Валиев А.К., Тепляков В.В., Мусаев Э.Р., Рогожин Д.В., Сушенцов Е.А., Мачак Г.Н. и соавт. Практические рекомендации по лечению первичных злокачественных опухолей костей. Злокачественные опухоли : Практические рекомендации RUSSCO #3s2, 2022 (том 12). 307–329.
13. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual, 8th edition. New York: Springer; 2017.
14. Casali PG, Bielack S, Abecassis N, et al.. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct 1;29(Suppl 4):iv79-95. PMID:30285218
15. Grimer R, Athanasou N, Gerrand C, et al. UK guidelines for the management of bone sarcomas. Sarcoma. 2010;2010:317462. PMID:21253474
16. Детская онкология. Национальное руководство. Под ред. М.Д. Алиева, В.Г. Полякова, Г.Л. Менткевича, С.А. Маяковой. М.: Издательская группа РОНЦ. Практическая медицина, 2012. 684 p.
17. Румянцев А.Г., Масчан А.А., Самочатова Е.В. Сопроводительная терапия и контроль инфекций при гематологических и онкологических заболеваниях. Москва: МЕДПРАКТИКА-М, 2009, 448 с.
18. Трахтман П.Е., Старостин Н.Н., Новичкова Г.А., Ворожцов И.Н. Трансфузионная терапия в клинической практике: учебно-методическое пособие дляя врачей, ординаторов, аспирантов / Национальный медицинский исследовательский центр детской гематологии, онкологии и иммунологии им. Д. Рогачева, Кафедра трансфузиологии и клинической лабораторной диагностики. Москва, 2021, 76 с.
19. Tal A.L., Doshi H., Parkar F., Abraham T., Love C., Ye K., et al. The Utility of 18FDG PET/CT Versus Bone Scan for Identi cation of Bone Metastases in a Pediatric Sarcoma Population and a Review of the Lit- erature. J Pediatr Hematol Oncol 2021; 43 (2): 52–8.
20. М.Я. Ядгаров, Е.Д. Киреева, Кайлаш, М.М. Дунайкин, Ю.Н. Ликарь. «Диагностическая роль ПЭТ/КТ с 18F-ФДГ и сцинтиграфии костей скелета у детей и молодых взрослых с костными саркомами: систематический обзор и метаанализ» Вопросы гематологии/онкологии и иммунопатологии в педиатрии. 2023;22(4):158-169 https://doi.org/10.24287/1726-1708-2023-22-4-158-169).
21. Трякин А.А., Бесова Н.С., Волков Н.М., Гладков О.А., Карасева В.В., Сакаева Д.Д. и соавт. Общие принципы проведения противоопухолевой лекарственной терапии. Практические рекомендации RUSSCO, часть 1. Злокачественные опухоли, 2023 (том 13), #3s2, стр. 28–41.
22. Peled Y, Levin D, Shiran S, Manisterski M, Shukrun R, Elhasid R. Prevalence and management of methotrexate-induced neurotoxicity in pediatric patients with osteosarcoma: a single-center experience. Int J Clin Oncol. 2022 Aug;27(8):1372-1378. doi: 10.1007/s10147-022-02184-y. Epub 2022 May 31. PMID: 35639227.
23. Lien HH, Blomlie V, Saeter G, Solheim O, Fosså SD. Osteogenic sarcoma: MR signal abnormalities of the brain in asymptomatic patients treated with high-dose methotrexate. Radiology. 1991 May;179(2):547-50. doi: 10.1148/radiology.179.2.2014309. PMID: 2014309.
24. Inaba H, Khan RB, Laningham FH, Crews KR, Pui CH, Daw NC. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol. 2008 Jan;19(1):178-84. doi: 10.1093/annonc/mdm466. Epub 2007 Oct 17. PMID: 17947226.
25. Латипова Д.Х., Андреев В.В., Маслова Д.А., Новик А.В., Проценко С.А. Неврологи- ческие осложнения противоопухолевой лекарственной терапии. Практические рекомендации RUSSCO, часть 2. Злокачественные опухоли, 2023 (том 13), #3s2, стр. 304–314
26. Tajino T, Kikuchi S, Yamada H, Takeda A, Konno S. Ifosfamide encephalopathy associated with chemotherapy for musculoskeletal sarcomas: incidence, severity, and risk factors. J Orthop Sci. 2010 Jan;15(1):104-11. doi: 10.1007/s00776-009-1431-y. Epub 2010 Feb 12. PMID: 20151259.
27. Malawer M.M., Sugarbaker P.H. Musculoskeletal Cancer Surgery - Treatment of Sarcomas and Allied Diseases. Springer, 2001. 626 p.
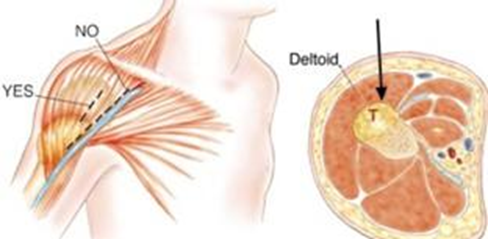
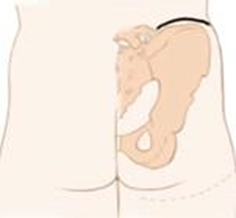
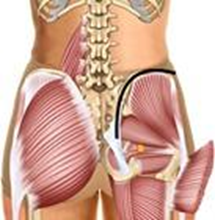
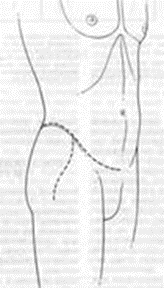
28. Traina F. et al. Current concepts in the biopsy of musculoskeletal tumors: AAOS exhibit selection // J. Bone Jt. Surg. - Am. Vol. Journal of Bone and Joint Surgery Inc., 2015. Vol. 97, № 2. P.e7(1).
29. Kubo T. et al. A meta-analysis supports core needle biopsy by radiologists for better histological diagnosis in soft tissue and bone sarcomas // Medicine (United States). Lippincott Williams and Wilkins, 2018. Vol. 97, № 29. P. e11567.
30. Мацко Д.Е. Саркомы костей: классификация, гистологическое строение, особенности морфологической диагностики // Практическая онкология. 2010. Vol. 11, № 1. P. 1–10.
31. Pohlig F. et al. Percutaneous core needle biopsy versus open biopsy in diagnostics of bone and soft tissue sarcoma: a retrospective study. // Eur. J. Med. Res. 2012. Vol. 17. P. 29.
32. Mankin H.J., Mankin C.J., Simon M.A. The hazards of the biopsy, revisited: For the members of the musculoskeletal tumor society // J. Bone Jt. Surg. - Ser. A. 1996. Vol. 78, № 5. P. 656–663.
33. Алиев М.Д. Злокачественные опухоли костей. Саркомы костей, мягких тканей и опухоли кожи 2010;2:3–8
34. Ritter J, Bielack S.S Osteosarcoma // Ann. Oncol. 2010. Vol 21. P. 320-325
35. Bielack S, Ju ̈rgens H, Jundt G et al. Osteosarcoma: the COSS experience. Cancer Treat Res 2009; 152: 289–308.
36. Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396-1408.
37. Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26(2):407-414.
38. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
39. EURAMOS-1 protocol, a randomized trial of the European and American Osteosarcoma Srudy Group to optimize treatment strategies for resectable osteosarcoma based on histological response to pre-operative chemotherapy. Appendix B.
40. Jaffe N, Keifer R 3rd, Robertson R, Cangir A, Wang A. Renal toxicity with cumulative doses of cis-diamminedichloroplatinum-II in pediatric patients with osteosarcoma. Effect on creatinine clearance and methotrexate excretion. Cancer. 1987 May 1;59(9):1577-81. doi: 10.1002/1097-0142(19870501)59:9<1577::aid-cncr2820590908>3.0.co;2-p. PMID: 3470110.
41. Laitinen M. et al. The prognostic and therapeutic factors which influence the oncological outcome of parosteal osteosarcoma // Bone Jt. J. British Editorial Society of Bone and Joint Surgery, 2015. Vol. 97B, № 12. P. 1698–1703.
42. Cesari M. et al. Periosteal osteosarcoma // Cancer. 2011. Vol. 117, № 8. P. 1731–1735.
43. Ruengwanichayakun P, Gambarotti M, Frisoni T, et al. Parosteal osteosarcoma: a monocentric retrospective analysis of 195 patients. Hum Pathol. 2019;91:11-18.
44. Grimer RJ, Bielack S, Flege S, et al. Periosteal osteosarcomaea European review of outcome. Eur J Cancer. 2005;41(18):2806-2811.
45. Gitelis S. et al. Principles of limb salvage surgery // Chapman`s Orthopaedic Surgery, 3rd edition. 2001. P. 3309–3381.
46. Marulanda G.A. et al. Use of extendable prostheses: A limb-salvaging alternative for patients with malignant bone tumors // Expert Review of Medical Devices. 2008. Vol. 5, № 4. P. 467–474.
47. Heck R.K. General principles of tumors // Campbells Operative Orthopaedics. 10th edition. 2004. P. 733–792.
48. Carrle D, Bielack S. Osteosarcoma lung metastases detection and principles of multimodal therapy. Cancer Treat Res. 2009;152:165-84. doi: 10.1007/978-1-4419-0284-9_8. PMID: 20213390.
49. Kempf-Bielack B, Bielack SS, Ju ̈rgens H et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol 2005; 20: 559–568.
50. Bielack SS, Kempf-Bielack B, Branscheid D et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive Cooperative Osteosarcoma Study Group patients. J Clin Oncol 2009; 27: 557–565.
51. Gaspar N, Campbell-Hewson Q, Huang J, Okpara CE, Bautista F. OLIE, ITCC-082: a Phase II trial of lenvatinib plus ifosfamide and etoposide in relapsed/refractory osteosarcoma. Future Oncol. 2021 Nov;17(32):4249-4261. doi: 10.2217/fon-2021-0743. Epub 2021 Aug 12. PMID: 34382412.
52. Gaspar N, Venkatramani R, Hecker-Nolting S, Melcon SG, Locatelli F, Bautista F, Longhi A, Lervat C, Entz-Werle N, Casanova M, Aerts I, Strauss SJ, Thebaud E, Morland B, Nieto AC, Marec-Berard P, Gambart M, Rossig C, Okpara CE, He C, Dutta L, Campbell-Hewson Q. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): a multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 2021 Sep;22(9):1312-1321. doi: 10.1016/S1470-2045(21)00387-9. Epub 2021 Aug 17. PMID: 34416158.
53. Palmerini E, Setola E, Grignani G, D'Ambrosio L, Comandone A, Righi A, Longhi A, Cesari M, Paioli A, Hakim R, Pierini M, Marchesi E, Vanel D, Pignochino Y, Donati DM, Picci P, Ferrari S. High Dose Ifosfamide in Relapsed and Unresectable High-Grade Osteosarcoma Patients: A Retrospective Series. Cells. 2020 Oct 31;9(11):2389. doi: 10.3390/cells9112389. PMID: 33142760; PMCID: PMC7692098.
54. Palmerini E, Jones RL, Marchesi E, Paioli A, Cesari M, Longhi A, Meazza C, Coccoli L, Fagioli F, Asaftei S, Grignani G, Tamburini A, Pollack SM, Picci P, Ferrari S. Gemcitabine and docetaxel in relapsed and unresectable high-grade osteosarcoma and spindle cell sarcoma of bone. BMC Cancer. 2016 Apr 20;16:280. doi: 10.1186/s12885-016-2312-3. PMID: 27098543; PMCID: PMC4839113.
55. Navid F, Willert JR, McCarville MB, et al. Combination of gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. Cancer 2008;113:419-425.
56. Song BS, Seo J, Kim DH, Lim JS, Yoo JY, Lee JA. Gemcitabine and docetaxel for the treatment of children and adolescents with recurrent or refractory osteosarcoma: Korea Cancer Center Hospital experience. Pediatr Blood Cancer. 2014 Aug;61(8):1376-81. doi: 10.1002/pbc.25035. Epub 2014 Apr 1. PMID: 24692087.
57. Raciborska A, Bilska K. Sorafenib in patients with progressed and refractory bone tumors. Med Oncol. 2018 Aug 16;35(10):126. doi: 10.1007/s12032-018-1180-x. PMID: 30116912; PMCID: PMC6097021.
58. Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an
Italian Sarcoma Group study. Ann Oncol 2012; 23:508-516.
59. Sugiyama M, Arakawa A, Shirakawa N, Tao K, Tanimura K, Nakajima M, Watanabe Y, Kumamoto T, Maniwa J, Yoneda A, Iwata S, Kobayashi E, Kawai A, Ogawa C. Safety and efficacy of multiple tyrosine kinase inhibitors in pediatric/adolescent and young adult patients with relapsed or refractory osteosarcomas: A singlei nstitution retrospective analysis. Pediatr Blood Cancer. 2023 Jul;70(7):e30360. doi: 10.1002/pbc.30360. Epub 2023 Apr 19. PMID: 37073613
60. Peretz Soroka H, Vora T, Noujaim J, Marcoux N, Cohen-Gogo S, Alcindor T, Holloway C, Rodrigues C, Karachiwala H, Alvi S, Lee U, Cheng S, Banerji S, Oberoi S, Feng X, Smrke A, Simmons C, Razak AA, Gupta AA. Real-world experience of tyrosine kinase inhibitors in children, adolescents and adults with relapsed or refractory bone tumours: A Canadian Sarcoma Research and Clinical Collaboration (CanSaRCC) study. Cancer Med. 2023 Sep;12(18):18872-18881. doi: 10.1002/cam4.6515. Epub 2023 Sep 19. PMID: 37724607; PMCID: PMC10557866.
61. Davis LE, Bolejack V, Ryan CW, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol 2019;37:1424-1431.
62. Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694-701.
63. Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol 2012;30:4148-4154.
64. Brennan B, Kirton L, Marec-Bérard P, Gaspar N, Laurence V, Martín-Broto J, Sastre A, Gelderblom H, Owens C, Fenwick N, Strauss S, Moroz V, Whelan J, Wheatley K. Comparison of two chemotherapy regimens in patients with newly diagnosed Ewing sarcoma (EE2012): an open-label, randomised, phase 3 trial. Lancet. 2022 Oct 29;400(10362):1513-1521. doi: 10.1016/S0140-6736(22)01790-1. PMID: 36522207.
65. Anderton J, Moroz V, Marec-Bérard P, Gaspar N, Laurence V, Martín-Broto J, Sastre A, Gelderblom H, Owens C, Kaiser S, Fernández-Pinto M, Fenwick N, Evans A, Strauss S, Whelan J, Wheatley K, Brennan B. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours - EURO EWING 2012 Protocol. Trials. 2020 Jan 17;21(1):96. doi: 10.1186/s13063-019-4026-8. PMID: 31952545; PMCID: PMC6969439.
66. International Randomised Controlled Trial for the Treatment of Newly Diagnosed Ewing's Sarcoma Family of Tumours. Euro Ewing 2012.
67. Романцова О.М. Интенсификация режимов индукционной химиотерапии у детей с саркомой Юинга: авторефер. Дис канд мед. Наук: ФГБУ «НМИЦ онкологии им. Н.Н. Блохина»; 2024
68. Ladenstein R. et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro- EWING 99 trial // J. Clin. Oncol. 2010. Vol. 28, № 20. P. 3284–3291.
69. Tenneti P. et al. Role of High-Dose Chemotherapy and Autologous Hematopoietic Cell Transplantation for Children and Young Adults with Relapsed Ewing’s Sarcoma: A Systematic Review
70. Whelan J, Le Deley MC, Dirksen U, Le Teuff G, Brennan B, Gaspar N, Hawkins DS, Amler S, Bauer S, Bielack S, Blay JY, Burdach S, Castex MP, Dilloo D, Eggert A, Gelderblom H, Gentet JC, Hartmann W, Hassenpflug WA, Hjorth L, Jimenez M, Klingebiel T, Kontny U, Kruseova J, Ladenstein R, Laurence V, Lervat C, Marec-Berard P, Marreaud S, Michon J, Morland B, Paulussen M, Ranft A, Reichardt P, van den Berg H, Wheatley K, Judson I, Lewis I, Craft A, Juergens H, Oberlin O; Euro-E.W.I.N.G.99 and EWING-2008 Investigators. High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol. 2018 Sep 6;36(31):JCO2018782516. doi: 10.1200/JCO.2018.78.2516. Epub ahead of print. PMID: 30188789; PMCID: PMC6209090.
71. Koscielniak E. et al. Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the German cooperative soft tissue sarcoma study CWS-86 // J. Clin. Oncol. American Society of Clinical Oncology, 1999. Vol. 17, № 12. P. 3706–3719.
72. Breneman J. et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: A report from the Children’S Oncology Group D9602 study // Int. J. Radiat. Oncol. Biol. Phys. Int J Radiat Oncol Biol Phys, 2012. Vol. 83, № 2. P. 720–726.
73. Gupta A, Riedel RF, Shah C, Borinstein SC, Isakoff MS, Chugh R, Rosenblum JM, Murphy ES, Campbell SR, Albert CM, Zahler S, Thomas SM, Trucco M. Consensus recommendations in the management of Ewing sarcoma from the National Ewing Sarcoma Tumor Board. Cancer. 2023 Nov 1;129(21):3363-3371. doi: 10.1002/cncr.34942. Epub 2023 Jul 5. PMID: 37403815.
74. Richey SL, Rao P, Wood CG, Patel S, Tannir NM. Metastatic extraosseous Ewing's sarcoma (EES)/primitive neuroectodermal tumor (PNET) of the kidney: 8-year durable response after induction and maintenance chemotherapy. Clin Genitourin Cancer. 2012 Sep;10(3):210-2. doi: 10.1016/j.clgc.2012.03.004. Epub 2012 Apr 13. PMID: 22503609; PMCID: PMC4121061.
75. Meyers PA, Ambati SR, Slotkin EK, Cruz FD, Wexler LH. The addition of cycles of irinotecan/temozolomide (i/T) to cycles of vincristine, doxorubicin, cyclophosphamide (VDC) and cycles of ifosfamide, etoposide (IE) for the treatment of Ewing sarcoma (ES) [abstract]. J Clin Oncol. 2018;36(15 suppl):10533. doi:10.1200/JCO.2018.36.15_ suppl.10533
76. Raciborska A, Bilska K, Rodriguez‐Galindo C. Maintenance treatment with trofosfamide in patients with primary bone Ewing sarcoma—single center experience. Dev Period Med. 2019;23:39‐44. doi:10.34763/devperiodmed.20192301.3944
77. Tamura A, Yamamoto N, Nino N, et al. Pazopanib maintenance therapy after tandem high‐dose chemotherapy for disseminated Ewing sarcoma. Int Cancer Conf J. 2019;8(3):95‐100. doi:10.1007/ s13691‐019‐00362‐w7
78. Minard-Colin V, Ichante JL, Nguyen L, Paci A, Orbach D, Bergeron C, Defachelles AS, André N, Corradini N, Schmitt C, Tabone MD, Blouin P, Sirvent N, Goma G, Geoerger B, Oberlin O. Phase II study of vinorelbine and continuous low doses cyclophosphamide in children and young adults with a relapsed or refractory malignant solid tumour: good tolerance profile and efficacy in rhabdomyosarcoma--a report from the Société Française des Cancers et leucémies de l'Enfant et de l'adolescent (SFCE). Eur J Cancer. 2012 Oct;48(15):2409-16. doi: 10.1016/j.ejca.2012.04.012. Epub 2012 May 25. PMID: 22633624.
79. Ju HY, Park M, Lee JA, Park HJ, Park SY, Kim JH, Kang HG, Yang HC, Park BK. Vincristine, Irinotecan, and Temozolomide as a Salvage Regimen for Relapsed or Refractory Sarcoma in Children and Young Adults. Cancer Res Treat. 2022 Apr;54(2):563-571. doi: 10.4143/crt.2021.178. Epub 2021 Jun 14. PMID: 34126703; PMCID: PMC9016305.
80. Büyükkapu Bay S, Kebudi R, Görgün O, Zülfikar B, Darendeliler E, Çakır FB. Vincristine, irinotecan, and temozolomide treatment for refractory/relapsed pediatric solid tumors: A single center experience. J Oncol Pharm Pract. 2019 Sep;25(6):1343-1348. doi: 10.1177/1078155218790798. Epub 2018 Aug 6. PMID: 30080131.
81. Raciborska A, Bilska K, Drabko K, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer 2013;60:1621-1625
82. Hunold A, Weddeling N, Paulussen M, et al. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer 2006;47:795-800.
83. Kushner BH, Kramer K, Meyers PA, et al. Pilot study of topotecan and high- dose cyclophosphamide for resistant pediatric solid tumors. Med Pediatr Oncol 2000;35:468-474.
84. Saylors RL 3rd, Stine KC, Sullivan J, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol 2001;19:3463-3469
85. Martin McCabe et al., Phase III assessment of topotecan and cyclophosphamide and high-dose ifosfamide in rEECur: An international randomized controlled trial of chemotherapy for the treatment of recurrent and primary refractory Ewing sarcoma (RR-ES).. JCO 40, LBA2-LBA2(2022). DOI:10.1200/JCO.2022.40.17_suppl.LBA2
86. Mori Y, Kinoshita S, Kanamori T, Kataoka H, Joh T, Iida S, Takemoto M, Kondo M, Kuroda J, Komatsu H. The Successful Treatment of Metastatic Extraosseous Ewing Sarcoma with Pazopanib. Intern Med. 2018 Sep 15;57(18):2753-2757. doi: 10.2169/internalmedicine.9879-17. Epub 2018 May 18. PMID: 29780156; PMCID: PMC6191593.
87. Attia S, Bolejack V, Ganjoo K, et al. A phase II trial of regorafenib in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC024 trial results. Cancer Med 2023;12:1532-1539.
88.Романцова О.М., Нисиченко Д.В., Хестанов Д.Б., Хайруллова В.В., Дзампаев А.З., Киргизов К.И. Лечение рецидивов саркомы Юинга у детей и подростков: современный взгляд. Российский журнал детской гематологии и онкологии (РЖДГиО). 2021.- Т.- 8, № 3. – С. 30- 42.
89. Юхта Т.В., Казанцев И.В., Геворгян А.Г. и др. Эффективность высокодозной полихимиотерапии с аутологич- ной трансплантацией гемопоэтических стволовых клеток в лечении детей и молодых взрослых с саркомой Юинга. Онкогематология – 2019. – Т. 14, № 4. – С. 47–58.
90. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of Denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 2013;14:901-8
91. Martin-Broto J, Cleeland CS, Glare PA, et al. Effects of Denosumab on pain and analgesic use in giant cell tumor of bone:interim results from a phase II study. Acta Oncol 2014;53:1173−9.
92. Reddy K, Ramirez L, Kukreja K, Venkatramani R. Response to Denosumab in 2 Children With Recurrent Giant Cell Tumor of the Bone With Pulmonary Metastasis. J Pediatr Hematol Oncol. 2021 Mar 1;43(2):e215-e218. doi: 10.1097/MPH.0000000000001654. PMID: 31714440.
93. Bercovitz RS., Josephson CD. Transfusion considerations in pediatric hematology and oncology patients. Hematol Oncol Clin North Am. 2016; 30(3): 695-709. doi: 10.106/j.hoc.2016.01.010.
94. Steiner ME, Zantek ND, Stanworth SJ, Parker RI, et al. Recommendations on RBC Transfusion Support in Children With Hematologic and Oncologic Diagnoses From the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018 Sep;19(9S Suppl 1): 149-156.
95. Shah N., Andrews J., Goodnough LT. Transfusions for anemia in adult and pediatric patients with malignancies. Blood Reviews. 2015; 29(5): 291-299. doi: 10.106/j.blre.2015.02.001
96.Nellis ME, Goel R, Karam O. Transfusion Management in Pediatric Oncology Patients. Hematol Oncol Clin North Am. 2019; 33(5): 903-913. doi: 10.1016/j.hoc.2019.05.011.
97.World Health Organization. (2021). Educational modules on clinical use of blood. https://www.who.int/publications/i/item/9789240033733
98.Долгов ВВ., Свирин ПВ. Лабораторная диагностика нарушений гемостаза. Москва: Триада, 2005 - с150. ISBN 5-94789-114-x.
99.Кречетова А.В. Нарушение гемостаза при сепсисе у онкогематологических больных c миелотоксическим агранулоцитозом: автореф. Дис канд мед. наук. М.: Гематологический научный центр; 2011
100.Kozek-langenecker S.A., Afshari A., Albaladejo P., et al. Management of severe perioperative bleeding Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013; 30: 270-382. DOI: 10.1097/ EJA.0b013e32835f4d5.
101.O'Shaughnessy D., Atterbury C., Bolton Maggs P., et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Hematol. 2004; 126(1): 11-28. DOI: 10.1111/j. 1365-2141,2004.04972.x.
102.Галстян Г.М., Гапонова Т.В., Жибурт Е.Б., Балашова Е.Н., Берковский А.Л., Быстрых О.А., Купряшов А.А., Оловникова Н.И., Ошоров А.В., Рыбка М.М., Троицкая В.В., Буланов А.Ю., Журавель С.В., Лубнин А.Ю., Мазурок В.А., Недомолкин С.В., Певцов Д.Э., Рогачевский О.В., Салимов Э.Л., Трахтман П.Е., Чжао А.В., Шерстнев Ф.С., Савченко В.Г. Клиническое использование криопреципитата. Гематология и трансфузиология. 2020; 65(1): 87-114. doi.org/10.35754/0234-5730-2020-65-1-87-114
103. Patel P. Interventions for the prevention of acute phase chemotherapy-induced nausea and vomiting in adult and pediatric patients: a systematic review and meta-analysis. // Patel P, Robinson PD, Wahib N [et al] // Support Care Cancer. 2022 Nov;30(11):8855-8869. doi: 10.1007/s00520-022-07287-w
104. Hesketh, P.J. Antiemetics: ASCO Guideline Update / P.J. Hesketh, M.G. Kris, E. Basch [et al.] // J Clin Oncol. – 2020. – JCO2001296. – doi:10.1200/JCO.20.01296.] и COG (Children’s Oncology Group)
105. Ramavath, D.N. Olanzapine for Prevention of Vomiting in Children and Adolescents Receiving Highly Emetogenic Chemotherapy: Investigator-Initiated, Randomized, Open-Label Trial / D.N. Ramavath, V. Sreenivas, S. Vishwajeet [et al.] // Journal of Clinical Oncology. – 2020. – Vol. 38 (32). – P. 3785-3793. – doi: 10.1200/JCO.20.00871;
106. Жуков, Н.В. Эффективность и безопасность малых доз оланзапина в профилактике тошноты и рвоты у детей и подростков, получающих высокоэметогенную химиотерапию. Промежуточные результаты рандомизированного исследования / Н.В. Жуков, Л.Л. Рабаева, Д.В. Литвинов // Вопросы гематологии/онкологии и иммунопатологии в педиатрии. – 2022. − №4. – С. 70–82. doi: 10.24287/1726-1708-2022-21-4-70-82
107. Owais Mohammed. Efficacy, safety and cost effectiveness of reduced-dose olanzapine versus aprepitant as a part of triple-antiemetic therapy in the prevention of chemotherapy induced nausea and vomiting / Owais Mohammed, Narender kumar Thota // Journal of Clinical Oncology - 2022. Vol. 40 (16) - doi:10.1200/JCO.2022.40.16_suppl.e24078;
108. Rudolph M.Navari. Olanzapine with or without an NK-1 receptor antagonist for preventing chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy: A phase III randomized, double-blind, placebo-controlled trial (ALLIANCE A221602). / Rudolph M. Navari, Jennifer Le-Rademacher, Fabrice Smieliauskas [et al] // - 2022. Vol. 40 (16) - https://doi.org/10.1200/JCO.2022.40.16_suppl.12107
109. Patil, V. Comparison of antiemetic efficacy and safety of palonosetron vs ondansetron in the prevention of chemotherapy-induced nausea and vomiting in children / V. Patil, H. Prasada, K. Prasad [et al.] // J Community Support Oncol. – 2015. – Vol. 13 (6). – P. 209-213. – doi: 10.12788/jcso.0139;
110. Chaudhary, N.K. Palonosetron is a Better Choice Compared with Ondansetron for the Prevention of Chemotherapy-induced Nausea and Vomiting (CINV) in a Resource-limited Pediatric Oncology Center: Results from a Randomized Control Trial / N.K. Chaudhary, R.R. John, D. Boddu [et al.] // J Pediatr Hematol Oncol. – 2019. – Vol. 41 (4). – P. 294-297. – doi: 10.1097/MPH.0000000000001357
111. Flank, J. Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-Induced Nausea and Vomiting in Children with Cancer / J. Flank, P.D. Robinson, M. Holdsworth [et al.] // Pediatr Blood Cancer. – 2016. – Vol. 63 (7). – P. 1144-1151. – doi:10.1002/pbc.25955
112. Langford P, Chrisp P. Fosaprepitant and aprepitant: an update of the evidence for their place in the prevention of chemotherapy-induced nausea and vomiting. Core Evid. 2010 Oct 21;5:77-90. doi: 10.2147/ce.s6012. PMID: 21042544; PMCID: PMC2963924/
113. Приказ МЗ РФ от 23.09.2020 №1008н «Об утверждении порядка обеспечения пациентов лечебным питанием»
114. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Oldervoll L, Ravasco P, Solheim TS, Strasser F, de van der Schueren M, Preiser JC, Bischoff SC. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr. 2021; 40(5):2898-2913. doi: 10.1016/j.clnu.2021.02.005
115. Вашура А.Ю. Нутритивная недостаточность, ее причины и пути коррекции у детей с опухолями ЦНС на этапе активной терапии и после ее окончания. Фарматека. 2019; 26 (10): 8–14
116. Парентеральное и энтеральное питание: национальное руководство / под ред. М.Ш. Хубутия, Т.С. Поповой, А.И. Салтанова. – М.: ГЭОТАР-Медиа, 2014. – 800с.
117. Mehta NM, Corkins MR, Lyman B, Malone A, Goday PS, Carney LN, Monczka JL, Plogsted SW, Schwenk WF; American Society for Parenteral and Enteral Nutrition Board of Directors. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37(4):460-81. doi: 10.1177/0148607113479972
118. Алымова Ю.А., Вашура А.Ю. Адекватная оценка нутритивного статуса в детской онкологии и гематологии - первый этап нутритивного сопровождения. Трудный пациент. 2019; Т. 17. № 8 (9). С. 54-59.
119. Martin L. et al. Diagnostic criteria for the classification of cancer-associated weight loss // J. Clin. Oncol. American Society of Clinical Oncology, 2015. Vol. 33, № 1. P. 90–99
120. Kondrup J. et al. ESPEN guidelines for nutrition screening 2002 // Clin. Nutr. Churchill Livingstone, 2003. Vol. 22, № 4. P. 415–421; Arends J. et al. ESPEN guidelines on nutrition in cancer patients // Clin. Nutr. Churchill Livingstone, 2017. Vol. 36, № 1. P. 11–48
121. Вашура АЮ, Кучер МА, Ковтун ТА, Алымова ЮA, Литвинов ДВ, Зубаровская ЛС, Кулагин АД. Роль и актуальность нутрициологического диагноза в онкопедиатрии. Медицинский Совет. 2023;(12):99-109
122. Yaprak D.S, Yalçın B., Pınar A.A., Büyükpamukçu M. Assessment of nutritional status in children with cancer: Significance of arm anthropometry and serum visceral proteins. Pediatr Blood Cancer. 2021;68(1):28752
123. Методические указания. Порядок проведения клинических исследований для оценки эффективности специализированной пищевой продукции диетического лечебного и диетического профилактического питания/ ФГБУН «ФИЦ питания и биотехнологии», Роспотребнадзор, Минздрав России, ФГБУН «ФНЦГ им. Ф.Ф.Эрисмана» Роспотребнадзора. - Москва. – 2023. – 28с.
124. Методическое руководство. Стандарты лечебного питания/ ФГБУН «ФИЦ питания и биотехнологии». - Москва. – 2017. – 338с.
125. Вашура А.Ю., Пятаева А.А., Карелин А.Ф. Питание и нутритивная поддержка детей со злокачественными новообразованиями после завершения лечения: основные аспекты. Вопросы детской диетологии. 2022; 20(6): 64–70
126. Rayar M., Webber C.E., Nayiager T., Sala A., Barr R.D. Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35:98-102; Pietila S., Makipernaa A., Sievanen H., Koivisto A.M., Wigren T., Lenko H.L. Obesity and metabolic changes are common in young childhood brain tumor survivors. Pediatr Blood Cancer. 2009;52:853-9
127. Joosten K, Embleton N, Yan W, Senterre T; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Energy. Clin Nutr. 2018;37(6 Pt B):2309-2314. doi:10.1016/j.clnu.2018.06.944
128. Особенности нутриционной поддержки больных в педиатрии. В кн.: Клиническое питание больных в интенсивной медицине: практическое руководство /под ред. Луфта В. М., Багненко С. Ф., издание второе, дополненное. СПб.: Арт-Экспресс, 2013–460.
129. Парентеральное и энтеральное питание детей. Практические рекомендации. Под ред. Ерпулевой Ю.В., Чубаровой А.И., Чугуновой Ю.Л. ГЭОТАР-Медиа, 2016г. 304с.; Arends J. et al. ESPEN guidelines on nutrition in cancer patients // Clin. Nutr. Churchill Livingstone, 2017. Vol. 36, № 1. P. 11–48
130. Современные рекомендации по питанию детей / под ред. Проф. Ю.Г. Мухиной, проф. И.Я. Коня. – М.: ИД «МЕДПРАКТИКА-М», 2010,568с.
131. Ерпулёва Ю. В. Парентеральное питание у детей Российский вестник детской хирургии, анестезиологии и реаниматологии. 2018; 8(1):49-56
132. Viani K., Trehan A., Manzoli B., Schoeman J. Assessment of nutritional status in children with cancer: A narrative review. Pediatr Blood Cancer. 2020;67 Suppl 3:e28211. https://doi: 10.1002/pbc.2821
133. Koletzko B., Goulet O., Hunt J., Krohn K., Shamir R. for the Parenteral Nutrition Guidelines Working Group. Guidelines on Paediatric Parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paeditric Research (ESPR). J. Pediatr. Gastroenterol.Nutr. 2005; 41: Suppl.2: S1S87. DOI: 10.1097/01. mpg.0000181841.07090.f4
134. L’Hotta AJ, Randolph SB, Reader B, Lipsey K, King AA. Clinical practice guideline and expert consensus recommendations for rehabilitation among children with cancer: A systematic review. CA Cancer J Clin. 2023; 73(5): 524-545. doi:10.3322/caac.21783
135. Fischmeister, G., Riedl, D., Sanio, G. et al. Rehabilitation for children and adolescents after cancer: importance and implementation in Austria. memo 14, 278–283 (2021). https://doi.org/10.1007/s12254-021-00729-x
136. Ospina PA, McComb A, Pritchard-Wiart LE, Eisenstat DD, McNeely ML. Physical therapy interventions, other than general physical exercise interventions, in children and adolescents before, during and following treatment for cancer. Cochrane Database Syst Rev. 2021 Aug 3;8(8):CD012924. doi: 10.1002/14651858.CD012924.pub2. PMID: 34343340; PMCID: PMC8407387.
137. Berrak SG, Pearson M, Berberoğlu S, Ilhan IE, Jaffe N. High-dose ifosfamide in relapsed pediatric osteosarcoma: therapeutic effects and renal toxicity. Pediatr Blood Cancer. 2005 Mar;44(3):215-9. doi: 10.1002/pbc.20228. PMID: 15503294.
138. Pawlowska AB, Sun V, Calvert GT, Karras NA, Sato JK, Anderson CP, Cheng JC, DiMundo JF, Femino JD, Lu J, Yang D, Dagis A, Miser JS, Rosenthal J. Long-Term Follow-up of High-Dose Chemotherapy with Autologous Stem Cell Transplantation in Children and Young Adults with Metastatic or Relapsed Ewing Sarcoma: A Single-Institution Experience. Transplant Cell Ther. 2021 Jan;27(1):72.e1-72.e7. doi: 10.1016/j.bbmt.2020.09.029. Epub 2020 Sep 29. PMID: 33007495
139. Fox E, Patel S, Wathen JK, et al. Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of Sarcoma Alliance for Research Through Collaboration Study 003. Oncologist. 2012;17:321. doi: 10.1634/theoncologist.2010-0265
140. Gosiengfiao Y, Reichek J, Woodman J, et al. Gemcitabine with or without docetaxel and resection for recurrent osteosarcoma: the experience at Children's Memorial Hospital. J Pediatr Hematol Oncol. 2012;34:e63–e65. doi: 10.1097/MPH.0b013e3182331ee8
141. He AN, Tang LN, Shen Z, et al. Efficacy and safety of gemcitabine-docetaxel combination therapy for recurrent or refractory high-grade osteosarcoma in China: a retrospective study of 18 patientб, s. Jpn J Clin Oncol. 2012;42:427–431. doi: 10.1093/jjco/hyr19
142. Wagner L, et al. , Pilot study of vincristine, oral irinotecan, and temozolomide (VOIT regimen) combined with bevacizumab in pediatric patients with recurrent solid tumors or brain tumors. Pediatr Blood Cancer, 2013. 60(9): p. 1447–51.
143. Chybicka A, Bogusławska-Jaworska J, Rosińska B, Wecławek-Tompol J, Armata J, Balcerska A, Balwierz W, Bubala H, Drabko K, Eliasinska A, Kedziora M, Sońta-Jakimczyk D, Sopylo B, Kołecki P, Kowalczyk J, Matysiak M, Rokicka-Milewska R, Stefaniak MJ, Stańczak E, Stencel D, Wysocki M, Płoszyńska A. G-CSF i GM-CSF w leczeniu neutropenii po chemioterapii nowotworów u dzieci [G-CSF and GM-CSF in treatment of neutropaenia after chemotherapy in children with neoplasms]. Med Wieku Rozwoj. 2000;4(1 Suppl 2):121-9. Polish. PMID: 12021471.
144. Inteernational randomized controlled trial of chemotherapy for the treatment of reccurent and primary refractory Ewing Sarcoma. Version 5.0. 2016
145. Haveman LM, van Ewijk R, van Dalen EC, Breunis WB, Kremer LC, van den Berg H, Dirksen U, Merks JH. High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents, and young adults with first recurrence of Ewing sarcoma. Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD011406. doi: 10.1002/14651858.CD011406.pub2. PMID: 34472084; PMCID: PMC8411193.
146. Koch R, Gelderblom H, Haveman L, Brichard B, Jürgens H, Cyprova S, van den Berg H, Hassenpflug W, Raciborska A, Ek T, Baumhoer D, Egerer G, Eich HT, Renard M, Hauser P, Burdach S, Bovee J, Bonar F, Reichardt P, Kruseova J, Hardes J, Kühne T, Kessler T, Collaud S, Bernkopf M, Butterfaß-Bahloul T, Dhooge C, Bauer S, Kiss J, Paulussen M, Hong A, Ranft A, Timmermann B, Rascon J, Vieth V, Kanerva J, Faldum A, Metzler M, Hartmann W, Hjorth L, Bhadri V, Dirksen U. High-Dose Treosulfan and Melphalan as Consolidation Therapy Versus Standard Therapy for High-Risk (Metastatic) Ewing Sarcoma. J Clin Oncol. 2022 Jul 20;40(21):2307-2320. doi: 10.1200/JCO.21.01942. Epub 2022 Apr 15. PMID: 35427190.
147. Жуков Н.В., Казакова Л.Л., Новичкова Г.А. Профилактика и лечение тошноты и рвоты у детей и подростков, получающих противоопухолевую терапию. Текущее состояние и потенциальные пути улучшения. Вопросы гематологии/онкологии и иммунопатологии в педиатрии. 2020;19(4):205-223. https://doi.org/10.24287/1726-1708-2020-19-4-205-223